Carnosine Attenuates the Development of both Type 2 Diabetes and Diabetic Nephropathy in BTBR ob/ob Mice - PubMed (original) (raw)
Maaike Schilperoort 2, Shiqi Zhang 1, Jana D Braun 1, Jiedong Qiu 1, Angelica Rodriguez 1, Diego O Pastene 1, Bernhard K Krämer 1, Hannes Köppel 1, Hans Baelde 2, Emile de Heer 2, Alessandra Anna Altomare 3, Luca Regazzoni 3, Alessandra Denisi 3, Giancarlo Aldini 3, Jacob van den Born 4, Benito A Yard 1, Sibylle J Hauske 1
Affiliations
- PMID: 28281693
- PMCID: PMC5345040
- DOI: 10.1038/srep44492
Carnosine Attenuates the Development of both Type 2 Diabetes and Diabetic Nephropathy in BTBR ob/ob Mice
Thomas Albrecht et al. Sci Rep. 2017.
Abstract
We previously demonstrated that polymorphisms in the carnosinase-1 gene (CNDP1) determine the risk of nephropathy in type 2 diabetic patients. Carnosine, the substrate of the enzyme encoded by this gene, is considered renoprotective and could possibly be used to treat diabetic nephropathy (DN). In this study, we examined the effect of carnosine treatment in vivo in BTBR (Black and Tan, BRachyuric) ob/ob mice, a type 2 diabetes model which develops a phenotype that closely resembles advanced human DN. Treatment of BTBR ob/ob mice with 4 mM carnosine for 18 weeks reduced plasma glucose and HbA1c, concomitant with elevated insulin and C-peptide levels. Also, albuminuria and kidney weights were reduced in carnosine-treated mice, which showed less glomerular hypertrophy due to a decrease in the surface area of Bowman's capsule and space. Carnosine treatment restored the glomerular ultrastructure without affecting podocyte number, resulted in a modified molecular composition of the expanded mesangial matrix and led to the formation of carnosine-acrolein adducts. Our results demonstrate that treatment with carnosine improves glucose metabolism, albuminuria and pathology in BTBR ob/ob mice. Hence, carnosine could be a novel therapeutic strategy to treat patients with DN and/or be used to prevent DN in patients with diabetes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
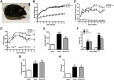
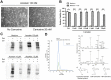
Figure 1. Carnosine attenuates diabetes in BTBR ob/ob mice.
(A) Representative image of a 24-week-old BTBR ob/ob mouse. (B) Body weight increased in ob/ob mice, independent of carnosine treatment. (C) Daily water intake was increased in ob/ob mice relative to wt/ob mice, and attenuated in carnosine-administered animals. (D) Weekly determination of fasting plasma glucose (FPG) indicated manifest hyperglycemia in ob/ob mice, which was reduced in carnosine-treated animals throughout the observation period. (E) Random glycemia (measured before perfusion) was significantly lower in carnosine-administered ob/ob mice compared with ob/ob controls. (F) At week 24 of age (18 weeks of treatment), HbA1c levels of ob/ob animals were elevated, and significantly reduced in carnosine treated mice. (G and H) Serum cholesterol and C-reactive protein (CRP) (at week 24 of age) were elevated in ob/ob mice, and unaffected by carnosine. Data represents means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared to ob/ob mice. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 compared to wt/ob mice. Car: carnosine.
Figure 2. Carnosine stimulates insulin secretion.
(A) Serum insulin levels (at week 24 of age) indicated hyperinsulinemia in ob/ob mice, which was further increased by more than twofold in carnosine-treated mice. (B) Glycemia correlated negatively with serum insulin levels in carnosine-administered mice. (C) C-peptide levels were significantly higher in carnosine-treated mice as compared to untreated ob/ob mice. (D) Glucagon levels were elevated in both ob/ob groups, but not affected by carnosine treatment. **P < 0.01, ***P < 0.001 compared to ob/ob mice. #P < 0.05, ##P < 0.01 compared to wt/ob mice. Car: carnosine.
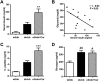
Figure 3. Carnosine protects from diabetic kidney damage.
(A) Carnosine administration for 18 weeks significantly reduced the ACR at week 24 by more than twofold compared with the ob/ob controls. (B) At the end of the experiment, kidney weight (average of both kidneys) was significantly increased in ob/ob mice, which was attenuated in carnosine-treated mice. (B and C) At the end of the experiment, the absolute kidney weight (average of both kidneys) was significantly increased in ob/ob mice. Carnosine treatment resulted in both reduced absolute and relative kidney weights. (D and E) Serum creatinine and blood urea nitrogen (BUN) were determined at the end of the experiment. The highest serum creatinine levels were found in heterozygous mice, being significantly different from the lowest levels found in carnosine-supplemented homozygous mice. No differences were observed with respect to BUN between any of the groups. Data represents means ± SEM. *P < 0.05, ****P < 0.0001 compared to ob/ob mice. ##P < 0.01, ####P < 0.0001 compared to wt/ob mice. Car: carnosine.
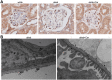
Figure 4. Carnosine reduces glomerular hypertrophy.
(A) Representative images of glomeruli (PAS-stained sections) from wt/ob, ob/ob and ob/ob carnosine-supplemented mice. (B,C and D) Surface areas of Bowman’s capsule (B), Bowman’s space (C) and the glomerular tuft (D) were increased in ob/ob mice, while carnosine treated mice showed a reduction in the area of Bowman’s capsule and space. (E) Surface area of Bowman’s space positively correlated with glycemia, HbA1c and albumin-creatinine ratio (ACR). Data represents means ± SEM. *P < 0.05 compared to ob/ob mice. ####P < 0.0001 compared to wt/ob mice. Car: carnosine.
Figure 5. Effect of carnosine on glomerular podocytes, glomerular ultrastructure and mesangial matrix expansion.
(A) Representative images of glomeruli immunostained for WT1 (and counterstained with hematoxylin) to visualize podocytes (brown) in wt/ob, ob/ob and ob/ob carnosine-supplemented mice. Podocyte loss observed in ob/ob mice was not prevented by carnosine treatment (quantification now shown). (B) Representative electron micrographs (30.000x) of glomeruli from ob/ob and ob/ob carnosine-supplemented mice. Untreated mice showed complete podocyte effacement (stars), GBM thickening and swelling of glomerular endothelial cells with loss of fenestrae (double arrows). Carnosine-treated mice displayed a normal capillary wall structure with intact podocyte foot processes, slit diaphragms (arrows) and endothelial fenestrae (arrowheads). FP: foot process; GBM: glomerular basement membrane; US: urinary space; CL: capillary lumen; Car: carnosine.
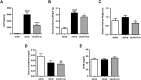
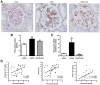
Figure 6. Effect of carnosine on fibronectin quantity in the mesangial matrix.
(A) Representative images of glomeruli immunostained for fibronectin (brown) and counterstained with hematoxylin in wt/ob, ob/ob and ob/ob carnosine-supplemented mice. (B) The quantity of fibronectin protein was increased in ob/ob mice compared to wt/ob controls. A trend was observed towards lower fibronectin content in carnosine-treated animals. (C) Fibronectin mRNA expression (shown relative to the wt/ob group) was significantly decreased in carnosine-treated mice compared to ob/ob controls. (D) The quantity of fibronectin protein positively correlated with glycemia, HbA1c and albumin-creatinine ratio (ACR). Data represents means ± SEM. *P < 0.05, ο_P_ = 0.09, θ_P_ = 0.058 compared to ob/ob mice. ##P < 0.01 compared to wt/ob mice. Car: carnosine.
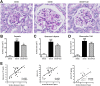
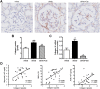
Figure 7. Effect of carnosine on the collagen quantity in the mesangial matrix.
(A) Representative images of glomeruli immunostained for collagen I (brown) and counterstained with hematoxylin in wt/ob, ob/ob and ob/ob carnosine-supplemented mice. (B) The quantity of collagen I protein was significantly higher in ob/ob control animals compared to wt/ob mice, and attenuated in carnosine-treated animals. (C) Collagen IV mRNA expression (shown relative to the wt/ob group) was significantly decreased in carnosine-treated mice as compared to ob/ob controls. (D) The amount of collagen I positively correlated with glycemia, HbA1c and albumin-creatinine ratio (ACR). Data represents means ± SEM. *P < 0.05, **P < 0.01 compared to ob/ob mice. #P < 0.05, ###P < 0.001 compared to wt/ob mice. Car: carnosine.
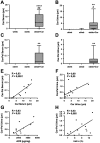
Figure 8. Carnosine and its acrolein adduct are increased in serum and urine of treated mice.
(A–D) Carnosine and carnosine-propanal levels are significantly increased in the serum and urine of treated mice compared to ob/ob mice (data are depicted in a box plot with whiskers extended to minimum and maximum values). (E,F) Carnosine levels in serum and urine of treated mice significantly correlated with the respective adduct levels in the same specimen. (G,H) The ACR of treated animals significantly correlated with urinary carnosine-propanal. HbA1c levels showed a similar trend, but this correlation was not statistically significant (P = 0.065). ****P < 0.0001, **P < 0.01 compared to ob/ob mice. ##P < 0.01, #P < 0.05 compared to wt/ob mice. Car: carnosine; Car-Pal: carnosine-propanal.
Figure 9. Carnosine abrogates acrolein toxicity in vitro by inhibition of protein carbonylation.
(A) Incubation of HUVECs with 130 nM of acrolein for 24 hours led to cell death, which was completely reversed when co-incubated with carnosine. Analogous results were observed for acrolein concentrations as low as 1,3 nM. (B) Quantitative MTT analysis (n = 6 for each condition) revealed complete cytotoxicity for acrolein concentrations of 1.3 nM to 130 μM. Carnosine co-incubation restored cell viability. (C) Representative OxyBlot results of lysed cells after 24 hours incubation with acrolein in presence or absence of carnosine. Signal intensity of bands representing carbonylated proteins was increased in acrolein stimulated cells. This increase was diminished in presence of carnosine. Samples without addition of derivation solution served as negative controls. (D) Identification of carnosine-acrolein Michael adduct in cell supernatants. 1) Single ion chromatograms of cell supernatants containing a fixed amount of carnosine (20 mM) and no acrolein (black line), 0.13 mM acrolein (orange line), 1.3 mM acrolein (green line) or 13 mM acrolein (blue line) reconstituted by setting the filter ion at m/z 283.14014. 2) Tandem mass spectrum of the ion at m/z 283.14014 detected in cell supernatants. 3) Tandem mass spectrum of a standard carnosine-acrolein Michael adduct. 4) Carnosine-acrolein adducts detected in cell supernatants. *P < 0.01, ****P < 0.0001. Car: carnosine. Deri: derivation solution.
Similar articles
- Effects of CP-900691, a novel peroxisome proliferator-activated receptor α, agonist on diabetic nephropathy in the BTBR ob/ob mouse.
Askari B, Wietecha T, Hudkins KL, Fox EJ, O'Brien KD, Kim J, Nguyen TQ, Alpers CE. Askari B, et al. Lab Invest. 2014 Aug;94(8):851-62. doi: 10.1038/labinvest.2014.80. Epub 2014 Jun 23. Lab Invest. 2014. PMID: 24955894 Free PMC article. - Carnosinase-1 overexpression, but not aerobic exercise training, affects the development of diabetic nephropathy in BTBR ob/ob mice.
Everaert I, He J, Hanssens M, Stautemas J, Bakker K, Albrecht T, Zhang S, Van der Stede T, Vanhove K, Hoetker D, Howsam M, Tessier FJ, Yard B, Baba SP, Baelde HJ, Derave W. Everaert I, et al. Am J Physiol Renal Physiol. 2020 Apr 1;318(4):F1030-F1040. doi: 10.1152/ajprenal.00329.2019. Epub 2020 Mar 9. Am J Physiol Renal Physiol. 2020. PMID: 32150446 - Human carnosinase 1 overexpression aggravates diabetes and renal impairment in BTBROb/Ob mice.
Qiu J, Albrecht T, Zhang S, Hauske SJ, Rodriguez-Niño A, Zhang X, Nosan D, Pastene DO, Sticht C, Delatorre C, van Goor H, Porubsky S, Krämer BK, Yard BA. Qiu J, et al. J Mol Med (Berl). 2020 Sep;98(9):1333-1346. doi: 10.1007/s00109-020-01957-0. Epub 2020 Aug 15. J Mol Med (Berl). 2020. PMID: 32803273 Free PMC article. - Carnosine and Diabetic Nephropathy.
Peters V, Yard B, Schmitt CP. Peters V, et al. Curr Med Chem. 2020;27(11):1801-1812. doi: 10.2174/0929867326666190326111851. Curr Med Chem. 2020. PMID: 30914013 Review. - [Carnosine, carnosinase and kidney diseases].
Kiliś-Pstrusińska K. Kiliś-Pstrusińska K. Postepy Hig Med Dosw (Online). 2012 Apr 20;66:215-21. doi: 10.5604/17322693.991600. Postepy Hig Med Dosw (Online). 2012. PMID: 22706107 Review. Polish.
Cited by
- L-Carnosine Stimulation of Coenzyme Q10 Biosynthesis Promotes Improved Mitochondrial Function and Decreases Hepatic Steatosis in Diabetic Conditions.
Schwank-Xu C, Forsberg E, Bentinger M, Zhao A, Ansurudeen I, Dallner G, Catrina SB, Brismar K, Tekle M. Schwank-Xu C, et al. Antioxidants (Basel). 2021 May 17;10(5):793. doi: 10.3390/antiox10050793. Antioxidants (Basel). 2021. PMID: 34067694 Free PMC article. - Effect of Carnosine or β-Alanine Supplementation on Markers of Glycemic Control and Insulin Resistance in Humans and Animals: A Systematic Review and Meta-analysis.
Matthews JJ, Dolan E, Swinton PA, Santos L, Artioli GG, Turner MD, Elliott-Sale KJ, Sale C. Matthews JJ, et al. Adv Nutr. 2021 Dec 1;12(6):2216-2231. doi: 10.1093/advances/nmab087. Adv Nutr. 2021. PMID: 34333586 Free PMC article. - Urinary Carnosinase-1 Excretion is Associated with Urinary Carnosine Depletion and Risk of Graft Failure in Kidney Transplant Recipients: Results of the TransplantLines Cohort Study.
Rodriguez-Niño A, Pastene DO, Post A, Said MY, Gomes-Neto AW, Kieneker LM, Heiner-Fokkema MR, Esatbeyoglu T, Rimbach G, Schnuelle P, Yard BA, Bakker SJL. Rodriguez-Niño A, et al. Antioxidants (Basel). 2021 Jul 9;10(7):1102. doi: 10.3390/antiox10071102. Antioxidants (Basel). 2021. PMID: 34356335 Free PMC article. - Carnosine scavenging of glucolipotoxic free radicals enhances insulin secretion and glucose uptake.
Cripps MJ, Hanna K, Lavilla C Jr, Sayers SR, Caton PW, Sims C, De Girolamo L, Sale C, Turner MD. Cripps MJ, et al. Sci Rep. 2017 Oct 17;7(1):13313. doi: 10.1038/s41598-017-13649-w. Sci Rep. 2017. PMID: 29042678 Free PMC article. - Carnosine Protects Macrophages against the Toxicity of Aβ1-42 Oligomers by Decreasing Oxidative Stress.
Caruso G, Benatti C, Musso N, Fresta CG, Fidilio A, Spampinato G, Brunello N, Bucolo C, Drago F, Lunte SM, Peterson BR, Tascedda F, Caraci F. Caruso G, et al. Biomedicines. 2021 Apr 26;9(5):477. doi: 10.3390/biomedicines9050477. Biomedicines. 2021. PMID: 33926064 Free PMC article.
References
- Gross J. L. et al.. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28, 164–176 (2005). - PubMed
- Bergrem H. & Leivestad T. Diabetic nephropathy and end-stage renal failure: the Norwegian story. Adv Ren Replace Ther 8, 4–12 (2001). - PubMed
- Gaede P., Lund-Andersen H., Parving H. H. & Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358, 580–591 (2008). - PubMed
- Krolewski M., Eggers P. W. & Warram J. H. Magnitude of end-stage renal disease in IDDM: a 35 year follow-up study. Kidney Int 50, 2041–2046 (1996). - PubMed
- Schena F. P. & Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16, S30–33 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous