Gender dimorphism of brain reward system volumes in alcoholism - PubMed (original) (raw)
Gender dimorphism of brain reward system volumes in alcoholism
Kayle S Sawyer et al. Psychiatry Res Neuroimaging. 2017.
Abstract
The brain's reward network has been reported to be smaller in alcoholic men compared to nonalcoholic men, but little is known about the volumes of reward regions in alcoholic women. Morphometric analyses were performed on magnetic resonance brain scans of 60 long-term chronic alcoholics (ALC; 30 men) and 60 nonalcoholic controls (NC; 29 men). We derived volumes of total brain, and cortical and subcortical reward-related structures including the dorsolateral prefrontal (DLPFC), orbitofrontal, and cingulate cortices, and the temporal pole, insula, amygdala, hippocampus, nucleus accumbens septi (NAc), and ventral diencephalon (VDC). We examined the relationships of the volumetric findings to drinking history. Analyses revealed a significant gender interaction for the association between alcoholism and total reward network volumes, with ALC men having smaller reward volumes than NC men and ALC women having larger reward volumes than NC women. Analyses of a priori subregions revealed a similar pattern of reward volume differences with significant gender interactions for DLPFC and VDC. Overall, the volume of the cerebral ventricles in ALC participants was negatively associated with duration of abstinence, suggesting decline in atrophy with greater length of sobriety.
Keywords: Abstinence; Alcohol; Brain morphometry; Drinking history; MRI; Reward network; Sex.
Copyright © 2017 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest: None of the authors reported having any relevant biomedical financial interest or potential conflicts of interest.
Figures
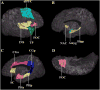
Figure 1
Three-dimensional representation of the cortical and subcortical structures of the brain's reward system. Image A shows a lateral view of the right hemisphere. Images B and C show a medial view of the right hemisphere; image D is an inferior view. Each region has been justified as an independent a priori analysis in previous literature that indicates involvement in alcoholism and addiction: Amyg – amygdala (32; 35; 68), CGa – anterior cingulate cortex (66; 69; 70), CGp – posterior cingulate cortex (71; 72), DLPFC – dorsolateral prefrontal cortex (35; 66; 69), FOC – orbitofrontal cortex (73–75), Hipp – hippocampus (29; 30), INS – insula (35; 66; 69; 70), NAc – nucleus accumbens septi (35), PHa – anterior parahippocampal gyrus (76), PHp – posterior parahippocampal gyrus (77), SC – subcallosal cortex (78), TP – temporal pole (79), VDC – ventral diencephalon (not shown; composed of the basal forebrain, hypothalamus, sublenticular extended amygdala, mammillary bodies, and a large portion of the ventral tegmentum area) (80). Modified from Makris et al. (2008), with permission.
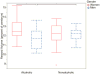
Figure 2
Reward volumes in alcoholic and nonalcoholic men and women. Lower alcoholism-related reward network volumes were observed for men, but increases were observed for women. Boxplot whiskers represent the most extreme values within double the interquartile range, and the middle band represents the median. There was one outlier, indicated by the red dot, and results did not change when that value was removed. Stars indicate significant differences (p < 0.05).
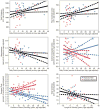
Figure 3
Brain volumes in relation to drinking variables. Brain volumes were significantly associated with daily drinks, duration of heavy drinking, and length of sobriety. These leverage plots (81) represent the relationships between the specified drinking history variables and regional brain volumes, covaried for age. For the anterior cingulate cortex and the temporal pole, the gender interactions were significant (p < 0.05), so the relationships were calculated separately for ALC men and ALC women, and the plots for men and women were overlaid. Red circles indicate ALC women; blue triangles indicate ALC men. Regression lines and 95% confidence curves of the slopes (dotted lines) are displayed.
Similar articles
- Decreased volume of the brain reward system in alcoholism.
Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Makris N, et al. Biol Psychiatry. 2008 Aug 1;64(3):192-202. doi: 10.1016/j.biopsych.2008.01.018. Epub 2008 Mar 28. Biol Psychiatry. 2008. PMID: 18374900 Free PMC article. - Regional Brain Volume Changes in Alcohol-Dependent Individuals During Short-Term and Long-Term Abstinence.
Zou X, Durazzo TC, Meyerhoff DJ. Zou X, et al. Alcohol Clin Exp Res. 2018 Jun;42(6):1062-1072. doi: 10.1111/acer.13757. Epub 2018 May 22. Alcohol Clin Exp Res. 2018. PMID: 29672876 Free PMC article. - Brain, behavioral, affective, and sex correlates of recovery from alcohol use disorders.
Thompson BL, Maleki N, Kelly JF, Sy KTL, Oscar-Berman M. Thompson BL, et al. Alcohol Clin Exp Res. 2021 Aug;45(8):1578-1595. doi: 10.1111/acer.14658. Epub 2021 Aug 25. Alcohol Clin Exp Res. 2021. PMID: 34432298 Free PMC article. - [A review of the neuroimaging studies of alcoholism].
Matsushita S, Higuchi S. Matsushita S, et al. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2007 Dec;42(6):615-21. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2007. PMID: 18240649 Review. Japanese. - Magnetic resonance imaging of the living brain: evidence for brain degeneration among alcoholics and recovery with abstinence.
Rosenbloom MJ, Pfefferbaum A. Rosenbloom MJ, et al. Alcohol Res Health. 2008;31(4):362-76. Alcohol Res Health. 2008. PMID: 23584010 Free PMC article. Review.
Cited by
- Cerebellar Morphometry and Cognition in the Context of Chronic Alcohol Consumption and Cigarette Smoking.
Cardenas VA, Hough CM, Durazzo TC, Meyerhoff DJ. Cardenas VA, et al. Alcohol Clin Exp Res. 2020 Jan;44(1):102-113. doi: 10.1111/acer.14222. Epub 2019 Nov 15. Alcohol Clin Exp Res. 2020. PMID: 31730240 Free PMC article. - At the intersection of alcohol use disorder and chronic pain.
Maleki N, Tahaney K, Thompson BL, Oscar-Berman M. Maleki N, et al. Neuropsychology. 2019 Sep;33(6):795-807. doi: 10.1037/neu0000558. Neuropsychology. 2019. PMID: 31448947 Free PMC article. Review. - Evaluating effects of sex and age on white matter microstructural alterations in alcohol use disorder: A diffusion tensor imaging study.
Kisner MA, Sussman L, Manuweera T, Grodin EN, Fede SJ, Sarlls JE, Momenan R. Kisner MA, et al. Alcohol Clin Exp Res. 2021 Sep;45(9):1790-1803. doi: 10.1111/acer.14678. Epub 2021 Aug 18. Alcohol Clin Exp Res. 2021. PMID: 34342014 Free PMC article. - Accelerated Aging of the Amygdala in Alcohol Use Disorders: Relevance to the Dark Side of Addiction.
Tomasi D, Wiers CE, Manza P, Shokri-Kojori E, Michele-Vera Y, Zhang R, Kroll D, Feldman D, McPherson K, Biesecker C, Schwandt M, Diazgranados N, Koob GF, Wang GJ, Volkow ND. Tomasi D, et al. Cereb Cortex. 2021 Jun 10;31(7):3254-3265. doi: 10.1093/cercor/bhab006. Cereb Cortex. 2021. PMID: 33629726 Free PMC article. - Hippocampal subfield volumes in abstinent men and women with a history of alcohol use disorder.
Sawyer KS, Adra N, Salz DM, Kemppainen MI, Ruiz SM, Harris GJ, Oscar-Berman M. Sawyer KS, et al. PLoS One. 2020 Aug 10;15(8):e0236641. doi: 10.1371/journal.pone.0236641. eCollection 2020. PLoS One. 2020. PMID: 32776986 Free PMC article.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. American Psychiatric Press; Washington, DC: 1994.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Statist Soc Series B (Methodological) 1995;57:289–300.
MeSH terms
Grants and funding
- R01 AG043640/AG/NIA NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- RF1 AG043640/AG/NIA NIH HHS/United States
- R21 AT008865/AT/NCCIH NIH HHS/United States
- K05 AA000219/AA/NIAAA NIH HHS/United States
- R01 AG042512/AG/NIA NIH HHS/United States
- R01 AA007112/AA/NIAAA NIH HHS/United States
- I01 CX000326/CX/CSRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical