Protein misfolding in neurodegenerative diseases: implications and strategies - PubMed (original) (raw)
Review
Protein misfolding in neurodegenerative diseases: implications and strategies
Patrick Sweeney et al. Transl Neurodegener. 2017.
Abstract
A hallmark of neurodegenerative proteinopathies is the formation of misfolded protein aggregates that cause cellular toxicity and contribute to cellular proteostatic collapse. Therapeutic options are currently being explored that target different steps in the production and processing of proteins implicated in neurodegenerative disease, including synthesis, chaperone-assisted folding and trafficking, and degradation via the proteasome and autophagy pathways. Other therapies, like mTOR inhibitors and activators of the heat shock response, can rebalance the entire proteostatic network. However, there are major challenges that impact the development of novel therapies, including incomplete knowledge of druggable disease targets and their mechanism of action as well as a lack of biomarkers to monitor disease progression and therapeutic response. A notable development is the creation of collaborative ecosystems that include patients, clinicians, basic and translational researchers, foundations and regulatory agencies to promote scientific rigor and clinical data to accelerate the development of therapies that prevent, reverse or delay the progression of neurodegenerative proteinopathies.
Keywords: Biomarkers; Chaperones; Drug discovery; Mouse models; Neurodegeneration; Proteostasis.
Figures
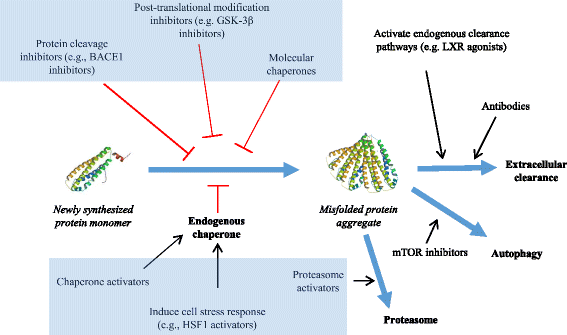
Fig. 1
Mechanisms involved in protein misfolding & therapeutic targets. A newly synthesized protein is stabilized by endogenous chaperone proteins. Under normal conditions abnormal protein aggregates (misfolded proteins) are degraded and/or cleared extracellularly, undergo autophagy or are degraded with the aid of the cellular proteasome. In cases of abnormality and misfolding of proteins (such as those present in many neurological diseases) post translational modification inhibitors, protein cleavage inhibitors and extrinsic molecular chaperones have been used in attempts to curtail or correct protein misfolding. In addition, post translational approaches to address and combat the presence of misfolded proteins include agonists that attempt to activate endogenous clearance pathways as well as the introduction of recombinant antibodies to work against the rogue protein
Similar articles
- The disturbance of protein synthesis/degradation homeostasis is a common trait of age-related neurodegenerative disorders.
Di Domenico F, Lanzillotta C. Di Domenico F, et al. Adv Protein Chem Struct Biol. 2022;132:49-87. doi: 10.1016/bs.apcsb.2022.05.008. Epub 2022 Jun 9. Adv Protein Chem Struct Biol. 2022. PMID: 36088079 - HSP70-HSP90 Chaperone Networking in Protein-Misfolding Disease.
Prodromou C, Aran-Guiu X, Oberoi J, Perna L, Chapple JP, van der Spuy J. Prodromou C, et al. Subcell Biochem. 2023;101:389-425. doi: 10.1007/978-3-031-14740-1_13. Subcell Biochem. 2023. PMID: 36520314 Review. - Unraveling the Role of Proteinopathies in Parasitic Infections.
Hurła M, Pikor D, Banaszek-Hurła N, Drelichowska A, Dorszewska J, Kozubski W, Kacprzak E, Paul M. Hurła M, et al. Biomedicines. 2025 Mar 3;13(3):610. doi: 10.3390/biomedicines13030610. Biomedicines. 2025. PMID: 40149586 Free PMC article. Review. - Potential Influence of Cyclo(His-Pro) on Proteostasis: Impact on Neurodegenerative Diseases.
Grottelli S, Costanzi E, Peirce MJ, Minelli A, Cellini B, Bellezza I. Grottelli S, et al. Curr Protein Pept Sci. 2018;19(8):805-812. doi: 10.2174/1389203719666180430155112. Curr Protein Pept Sci. 2018. PMID: 29708066 Review. - Molecular Chaperones: A Double-Edged Sword in Neurodegenerative Diseases.
Tittelmeier J, Nachman E, Nussbaum-Krammer C. Tittelmeier J, et al. Front Aging Neurosci. 2020 Oct 6;12:581374. doi: 10.3389/fnagi.2020.581374. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33132902 Free PMC article. Review.
Cited by
- S-nitrosylated PARIS Leads to the Sequestration of PGC-1α into Insoluble Deposits in Parkinson's Disease Model.
Kim H, Lee JY, Park SJ, Kwag E, Kim J, Shin JH. Kim H, et al. Cells. 2022 Nov 19;11(22):3682. doi: 10.3390/cells11223682. Cells. 2022. PMID: 36429110 Free PMC article. - The therapeutic potential of triptolide and celastrol in neurological diseases.
Cui Y, Jiang X, Feng J. Cui Y, et al. Front Pharmacol. 2022 Oct 19;13:1024955. doi: 10.3389/fphar.2022.1024955. eCollection 2022. Front Pharmacol. 2022. PMID: 36339550 Free PMC article. Review. - The Antioxidative Effects of Picein and Its Neuroprotective Potential: A Review of the Literature.
Elyasi L, Rosenholm JM, Jesmi F, Jahanshahi M. Elyasi L, et al. Molecules. 2022 Sep 21;27(19):6189. doi: 10.3390/molecules27196189. Molecules. 2022. PMID: 36234724 Free PMC article. Review. - Upregulated Expression of MicroRNA-204-5p Leads to the Death of Dopaminergic Cells by Targeting DYRK1A-Mediated Apoptotic Signaling Cascade.
Chiu CC, Yeh TH, Chen RS, Chen HC, Huang YZ, Weng YH, Cheng YC, Liu YC, Cheng AJ, Lu YC, Chen YJ, Lin YW, Hsu CC, Chen YL, Lu CS, Wang HL. Chiu CC, et al. Front Cell Neurosci. 2019 Sep 13;13:399. doi: 10.3389/fncel.2019.00399. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31572127 Free PMC article. - BAG2 drives chemoresistance of breast cancer by exacerbating mutant p53 aggregate.
Huang X, Shi D, Zou X, Wu X, Huang S, Kong L, Yang M, Xiao Y, Chen B, Chen X, Ouyang Y, Song L, Jian Y, Lin C. Huang X, et al. Theranostics. 2023 Jan 1;13(1):339-354. doi: 10.7150/thno.78492. eCollection 2023. Theranostics. 2023. PMID: 36593950 Free PMC article.
References
- Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nature reviews. Mol Cell Biol. 2014;15:384–396. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous