Phase Separation of C9orf72 Dipeptide Repeats Perturbs Stress Granule Dynamics - PubMed (original) (raw)
. 2017 Mar 16;65(6):1044-1055.e5.
doi: 10.1016/j.molcel.2017.02.013.
Elke Bogaert 1, Denes Kovacs 2, Albert Konijnenberg 3, Evy Timmerman 4, Alex Volkov 2, Mainak Guharoy 2, Mathias De Decker 1, Tom Jaspers 1, Veronica H Ryan 5, Abigail M Janke 6, Pieter Baatsen 7, Thomas Vercruysse 8, Regina-Maria Kolaitis 9, Dirk Daelemans 8, J Paul Taylor 9, Nancy Kedersha 10, Paul Anderson 10, Francis Impens 4, Frank Sobott 11, Joost Schymkowitz 12, Frederic Rousseau 12, Nicolas L Fawzi 6, Wim Robberecht 13, Philip Van Damme 14, Peter Tompa 15, Ludo Van Den Bosch 16
Affiliations
- PMID: 28306503
- PMCID: PMC5364369
- DOI: 10.1016/j.molcel.2017.02.013
Phase Separation of C9orf72 Dipeptide Repeats Perturbs Stress Granule Dynamics
Steven Boeynaems et al. Mol Cell. 2017.
Abstract
Liquid-liquid phase separation (LLPS) of RNA-binding proteins plays an important role in the formation of multiple membrane-less organelles involved in RNA metabolism, including stress granules. Defects in stress granule homeostasis constitute a cornerstone of ALS/FTLD pathogenesis. Polar residues (tyrosine and glutamine) have been previously demonstrated to be critical for phase separation of ALS-linked stress granule proteins. We now identify an active role for arginine-rich domains in these phase separations. Moreover, arginine-rich dipeptide repeats (DPRs) derived from C9orf72 hexanucleotide repeat expansions similarly undergo LLPS and induce phase separation of a large set of proteins involved in RNA and stress granule metabolism. Expression of arginine-rich DPRs in cells induced spontaneous stress granule assembly that required both eIF2α phosphorylation and G3BP. Together with recent reports showing that DPRs affect nucleocytoplasmic transport, our results point to an important role for arginine-rich DPRs in the pathogenesis of C9orf72 ALS/FTLD.
Keywords: FUS; LLPS; amyotrophic lateral sclerosis; frontotemporal lobar degeneration; hnRNP; intrinsically disordered protein; low complexity domain; phase transition; prion-like domain; protein aggregation.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
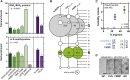
Arginine-Rich Motifs Are Enriched in Cellular Liquid-like Compartments and Can Phase Separate In Vitro (A) Proteins in ribonucleoprotein complexes (Castello et al., 2012, Topisirovic et al., 2009), liquid compartments (Andersen et al., 2005, Jain et al., 2016, Liu et al., 2010), or proteins with prion-like domains (Kato et al., 2012, March et al., 2016) are enriched for both tri-RGG/di-RGG (Thandapani et al., 2013) and R motifs (Mitrea et al., 2016). Binomial test. (B) Balloon plot indicating sequence redundancy of RGG boxes. (C) Synthetic RGG boxes and arginine-rich C9orf72 DPRs phase separate following the addition of a molecular crowder. Measured at room temperature (RT), mean ± SD is depicted. The extent of phase separation is correlated with arginine content (Spearman). (D) Examples of RGG and DPR phase separation. Pictures taken at 4°C. Scale bar represents 10 μm. See also Figure S1.
Figure 2
PR20 Peptide Undergoes Liquid-Liquid Phase Separation (A) A solution of 1 mM PR20 with 20% PEG is clear at room temperature, but phase separates after cooling. (B) Demixed PR20 droplets dissolve following a temperature increase. (C) PR20 droplets are highly circular suggesting surface tension minimization (250 μM PR20, 30% PEG, RT). (D) Large PR droplets deform when shear stress is applied (arrows), but take up a circular shape after stress relief as indicated by an aspect ratio approximating one (250 μM PR20, 30% PEG, RT). (E) Volume is conserved following the fusion of two PR20 droplets (250 μM PR20, 30% PEG, RT). Scale bars represent 10 μm.
Figure 3
Molecular Determinants of PR20 Phase Separation (A) PR20 LLPS is dependent on molecular crowding. (B) PR LLPS is length dependent. (C) Addition of 1,6-hexanediol does not affect PR20 LLPS. (D) Addition of NaCl inhibits PR20 LLPS. (E and E’) PR-PEG LLPS is dependent on inorganic counteranions and correlated to anionic charge. (F and F’) RNA dose dependently induces PR20 LLPS in the absence of molecular crowder. (G and G’) Addition of PR20 clusters poly-tyrosine polymers in the absence of molecular crowder. Depict schemes of molecular interactions and phase contrast images of solutions (E’, F’, and G’). Mean ± SD, n = 3 (A–G). All measurements at RT. Scale bars represent 20 μm. See also Figures S1 and S2.
Figure 4
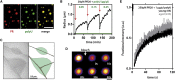
PR-RNA Droplets Are Dynamic Liquid Compartments (A) Fluorescently tagged PR20 and polyU RNA colocalize in phase separated droplets. Scale represents 10 μm. (B) Spontaneous fusion behavior of PR20-polyU RNA droplets over time, as expressed by changes in hydrodynamic radius over time as measured by DLS. Successive additions of polyU RNA indicated by red arrows. Final RNA concentration (green). The radius of polyU is 22nm (green line). (C) Cryo-TEM of two PR20-polyU droplets (green) wetting carbon support (brown). No obvious structures can be observed within the droplets. (D) Full droplet bleach of PR20 fluorescence indicates exchange between droplet and solution. Scale represents 5 μm. (E) Within-droplet bleach on old versus young droplets indicates that PR20-polyU droplets do not mature or age. Mean ± SD, n = 12 droplets. See also Figure S2.
Figure 5
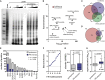
PR30 Initiates Phase Separation of RNA Granule Components and Metastable Proteins In Vitro (A) Addition of PR30 to cleared cell lysate induces LLPS with the formation of an insoluble fraction. Background from pre-cleared lysate not treated with PR was effectively zero. Weak interactions can be stabilized by mild PFA crosslinking. (B) The PR30 interactome (n = 874) is enriched for GO biological processes centered on RNA and protein metabolism. Benjamini-Hochberg. (C and D) The PR30 interactome significantly overlaps with cellular liquid compartments (Andersen et al., 2005, Jain et al., 2016) (C) and metastable proteins (Kato et al., 2012, Olzscha et al., 2011) (D). Binomial test. (E and F) The PR30 interactome is enriched for RNA binding domains (E) and arginine-rich motifs (Mitrea et al., 2016) (F). Binomial test. (G and H) The PR30 interactome is enriched for disordered (G) and supersaturated proteins (Ciryam et al., 2013) (H). Boxplots, whiskers indicate range. Mann-Whitney. See also Figures S3–S5 and Table S1.
Figure 6
PR100 Expression Alters Stress Granule Dynamics in Cells (A) Expression of PR100 in HeLa cells induces cytoplasmic SGs assembly positive for PR. (B) PR100 is more efficient at inducing SGs than PA100. (C) Arsenite and puromycin increase number of SGs in PR100 transfected cells, whereas cyclohexamide decreases them. (D) Non-phosphorylatable form (AA) of eIF2α prevents PR100-induced SG assembly in MEFs. (E) G3BP1/2 knockout prevents PR100 induced SG assembly in U2OS cells. (F) PR100-induced SGs have a mildly reduced FRAP recovery for G3BP1 compared to arsenite-induced ones. Repeated-measures ANOVA. Mean ± SEM, n = 17 SGs. (G) SG enrichment of several RNA binding proteins is altered in PR100-induced SGs compared to arsenite-induced ones. Boxplots, whiskers indicate range. Mann-Whitney. n > 60 SGs, from three independent experiments. Fischer’s exact test. n ≥ 100 cells, from three independent experiments (A–E). See also Figure S6.
Figure 7
PR30 Affects Phase Transitions of PrLDs (A) FUS LC droplets spontaneously fuse over time into larger droplets, as characterized by an increase in droplet size. This spontaneous fusion behavior is prevented by addition of PR30, but not GP30. Boxplots, whiskers indicate range. Kruskal-Wallis. n = (318–3,349) droplets. 50 mM MES buffer, 200 μM NaCl. (B) The FUS LC domain (amino acids [aa] 1–163) poorly phase separates, but addition of PR30 increases phase separation dose dependently. (C) Kinetic analysis of ThT fluorescence over a 12-hr period for different FUS LC-PR30 combinations. Addition of PR increases ThT fluorescence. Of note, PR30 by itself did not yield any increase in ThT fluorescence over time (data not shown). Mean ± SD (B and C). See also Figure S7.
Similar articles
- C9orf72-linked arginine-rich dipeptide repeats aggravate pathological phase separation of G3BP1.
Van Nerom M, Ahmed J, Lazar T, Meszaros A, Galand Q, De Malsche W, Van Lindt J, Pancsa R, Maes D, Tompa P. Van Nerom M, et al. Proc Natl Acad Sci U S A. 2024 Dec 10;121(50):e2402847121. doi: 10.1073/pnas.2402847121. Epub 2024 Dec 2. Proc Natl Acad Sci U S A. 2024. PMID: 39621905 Free PMC article. - C9orf72 Dipeptide Repeats Impair the Assembly, Dynamics, and Function of Membrane-Less Organelles.
Lee KH, Zhang P, Kim HJ, Mitrea DM, Sarkar M, Freibaum BD, Cika J, Coughlin M, Messing J, Molliex A, Maxwell BA, Kim NC, Temirov J, Moore J, Kolaitis RM, Shaw TI, Bai B, Peng J, Kriwacki RW, Taylor JP. Lee KH, et al. Cell. 2016 Oct 20;167(3):774-788.e17. doi: 10.1016/j.cell.2016.10.002. Cell. 2016. PMID: 27768896 Free PMC article. - c-Jun N-Terminal Kinase Promotes Stress Granule Assembly and Neurodegeneration in C9orf72-Mediated ALS and FTD.
Sahana TG, Chase KJ, Liu F, Lloyd TE, Rossoll W, Zhang K. Sahana TG, et al. J Neurosci. 2023 Apr 26;43(17):3186-3197. doi: 10.1523/JNEUROSCI.1799-22.2023. Epub 2023 Apr 4. J Neurosci. 2023. PMID: 37015810 Free PMC article. - Pathogenesis underlying hexanucleotide repeat expansions in C9orf72 gene in amyotrophic lateral sclerosis.
Chong ZZ, Menkes DL, Souayah N. Chong ZZ, et al. Rev Neurosci. 2023 Aug 2;35(1):85-97. doi: 10.1515/revneuro-2023-0060. Print 2024 Jan 29. Rev Neurosci. 2023. PMID: 37525497 Review.
Cited by
- Altered Phase Separation and Cellular Impact in _C9orf72_-Linked ALS/FTD.
Solomon DA, Smikle R, Reid MJ, Mizielinska S. Solomon DA, et al. Front Cell Neurosci. 2021 Apr 21;15:664151. doi: 10.3389/fncel.2021.664151. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33967699 Free PMC article. - Phase transition of RNA-protein complexes into ordered hollow condensates.
Alshareedah I, Moosa MM, Raju M, Potoyan DA, Banerjee PR. Alshareedah I, et al. Proc Natl Acad Sci U S A. 2020 Jul 7;117(27):15650-15658. doi: 10.1073/pnas.1922365117. Epub 2020 Jun 22. Proc Natl Acad Sci U S A. 2020. PMID: 32571937 Free PMC article. - Molecular Dissection of FUS Points at Synergistic Effect of Low-Complexity Domains in Toxicity.
Bogaert E, Boeynaems S, Kato M, Guo L, Caulfield TR, Steyaert J, Scheveneels W, Wilmans N, Haeck W, Hersmus N, Schymkowitz J, Rousseau F, Shorter J, Callaerts P, Robberecht W, Van Damme P, Van Den Bosch L. Bogaert E, et al. Cell Rep. 2018 Jul 17;24(3):529-537.e4. doi: 10.1016/j.celrep.2018.06.070. Cell Rep. 2018. PMID: 30021151 Free PMC article. - FAM69C functions as a kinase for eIF2α and promotes stress granule assembly.
Wu Z, Mei F, Gan Y, Liu A, Hu J, Jin Y, Yin Y. Wu Z, et al. EMBO Rep. 2023 May 4;24(5):e55641. doi: 10.15252/embr.202255641. Epub 2023 Mar 16. EMBO Rep. 2023. PMID: 36929224 Free PMC article. - Optical control of protein delivery and partitioning in the nucleolus.
Tan P, Hong T, Cai X, Li W, Huang Y, He L, Zhou Y. Tan P, et al. Nucleic Acids Res. 2022 Jul 8;50(12):e69. doi: 10.1093/nar/gkac191. Nucleic Acids Res. 2022. PMID: 35325178 Free PMC article.
References
- Aguzzi A., Altmeyer M. Phase separation: linking cellular compartmentalization to disease. Trends Cell Biol. 2016;26:547–558. - PubMed
- Andersen J.S., Lam Y.W., Leung A.K., Ong S.E., Lyon C.E., Lamond A.I., Mann M. Nucleolar proteome dynamics. Nature. 2005;433:77–83. - PubMed
- Ash P.E., Bieniek K.F., Gendron T.F., Caulfield T., Lin W.L., Dejesus-Hernandez M., van Blitterswijk M.M., Jansen-West K., Paul J.W., 3rd, Rademakers R. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77:639–646. - PMC - PubMed
- Aumiller W.M., Jr., Keating C.D. Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat. Chem. 2016;8:129–137. - PubMed
MeSH terms
Substances
Grants and funding
- T32 MH020068/MH/NIMH NIH HHS/United States
- R01 GM111700/GM/NIGMS NIH HHS/United States
- P20 GM104937/GM/NIGMS NIH HHS/United States
- R35 NS097974/NS/NINDS NIH HHS/United States
- R01 GM118530/GM/NIGMS NIH HHS/United States
- 647458/ERC_/European Research Council/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous