How Do J-Proteins Get Hsp70 to Do So Many Different Things? - PubMed (original) (raw)
Review
How Do J-Proteins Get Hsp70 to Do So Many Different Things?
Elizabeth A Craig et al. Trends Biochem Sci. 2017 May.
Abstract
Hsp70 chaperone machineries have pivotal roles in an array of fundamental biological processes through their facilitation of protein folding, disaggregation, and remodeling. The obligate J-protein co-chaperones of Hsp70s drive much of this remarkable multifunctionality, with most Hsp70s having multiple J-protein partners. Recent data suggest that J-protein-driven versatility is substantially due to precise localization within the cell and the specificity of substrate protein binding. However, this relatively simple view belies the intricacy of J-protein function. Examples are emerging of J-protein interactions with Hsp70s and other chaperones, as well as integration into broader cellular networks. These interactions fine-tune, in critical ways, the ability of Hsp70s to participate in diverse cellular processes.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
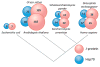
Figure I. Variation of J-protein and Hsp70 gene number across model organisms
Best estimates of gene numbers for each organism are given, and represented by the size of the circle [78, 79].
Figure I. Double β-barrel domain J-proteins
Structures of double β-barrel domains of S. cerevisiae J-proteins Ydj1 (left; PDB: 1NLT), with yellow balls indicting Zinc ions) and Sis1 (right, PDB: 1C3G); generated using PyMOL software (
). Examples of members of the two classes from Escherichia coli (Ec), Saccharomyces cerevisiae (Sc) and Homo sapiens (Hs), ZnBD present or absent, left and right, respectively, are given in boxes.
Figure 1. J-proteins function in many cellular processes
J-proteins are found in major cellular compartments: endoplasmic reticulum (ER, blue), cytosol/nucleus (Cyt/Nuc, purple) and mitochondria (Mito, orange), as indicated by color-coded key at bottom-left. (Top) An overview of cellular processes in which J-protein/Hsp70 chaperone systems function, with the adjacent small circles indicating the compartments in which the process occurs. Those processes listed on the left half are, in general, carried out by J-proteins referred to in the text as “general binders”, those that interact with a wide range of substrates. The ER and mitochondrial process of “Protein translocation across membranes” (middle right) is driven by J-proteins that are localized to a specific site of action where many different substrates emerge from the membrane. Remodeling of protein complexes (far right, top and bottom), generally involve “specific binders”, which interact with one or a few substrates and function in a specific cellular process, as discussed in the text. However, it should be kept in mind that all these generalizations have exceptions and there are cases that are not easily classified. For example, amorphous protein aggregates and yeast prions can be considered protein complexes, but both are disassembled, in part, through the action of the “broad binder” β-barrel J-proteins.
Figure 2. Hsp70 machinery fundamentals
Hsp70’s two domain architecture [N-terminal nucleotide binding domain (NBD) and C-terminal substrate binding domain (SBD)] is key to its function, as is interaction of J-protein and nucleotide exchange factor (NEF) co-chaperones, which, as indicated, stimulate ATPase activity and exchange of nucleotide, respectively. This figure also serves to illustrate four functional keys to the interaction of Hsp70 with substrate: (1) Dramatic difference between the ATP- and ADP-bound conformations are key to substrate interaction on a biologically relevant timescale. When ATP is bound, the SBD is docked onto the NBD (left). When ADP is bound the two domains do not interact, tethered to each other only by a linker (right). The SBD contains two subdomains: one containing the substrate-binding cleft, the other a lid. Both subdomains, as well as the linker, interact with the NBD in the docked, ATP state. The lid is restrained by this interaction, giving substrate easy, rapid access to the cleft. In the ADP-state, when closed, the lid limits access of substrate to the cleft, but once bound stabilizes it. (2) The two conformational states are not static, stable states, but are dynamic, with intermediates, such as the one shown in brackets with the lid undocked, but linker and cleft subdomain docked (center). (3) NBD’s ATPase activity serves as a switch between conformations. ATPase stimulation is the core activity of J-proteins. The J-domain (J) forms a finger-like structure that interacts at the interface between the NBD and SBD. Substrate interaction in the peptide binding cleft also stimulates Hsp70s ATPase activity, with coordinated timing of stimulation with the J-domain likely being key to productive binding of substrate (center and right). (4) NEFs are also key to regulating the cycle by stimulating release of ADP, allowing binding of ATP, which is typically more abundant (bottom).
Figure 3. Diverse modes of binding of substrate by J-proteins
J-proteins have structurally diverse substrate binding domains, which range in binding specificity from (A) very selective (i.e. in some cases a single substrate), that is “specific binders” to (B) quite promiscuous, binding to most any short hydrophobic stretch of amino acids, that is “broad binders”. The 4 “broad binders” of the human ER lumen are shown. ERdj3 is a double β-barrel protein (see Box 3). ERdj4 has an ill-defined substrate binding domain (S-binding). Erdj5 and ERdj6 have thioredoxin repeats (TRX) with reductase activity and tetratricopeptide repeats (TPR), respectively, but exact sites of substrate binding are yet to be well defined. (C) Many J-proteins do not fall neatly into these two categories. The DNAJB6/8 J-proteins that bind polyglutamine (polyQ) stretches are an example of the less well-defined intermediate class; the serine/threonine-rich (S/T) region is the experimentally defined region critical for polyQ binding, but it is dispensable for binding of another substrate, as described in the text. As the J-protein family as a whole becomes better characterized, well defined classes of J-proteins based on binding specificity will likely be difficult to delineate, as the binding specificity spectrum is likely a continuous one. Most common names of J-proteins S. cerevisiae (Sc) and H. sapiens (Hs) used in the literature are listed. Domain organization of representative J-proteins is drawn to scale, except where indicated by hatch (//) marks; J- J-domain; G/F- glycine/phenylalanine rich region; Cys-cysteine-rich region.
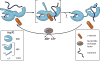
Figure 4. Localization of J-proteins to specific sites within a compartment
J-proteins that localize (A) near the opening ribosome exit tunnel and (B) at the import channel (translocon) in the ER and inner mitochondrial membranes do not bind substrates, but help recruit and orient their partner Hsp70 for effective substrate binding. (A) One ribosome-associated J-protein, called Zuo1/Zuotin, interacts with both subunits via indicated protein (eL31, yellow) and rRNAs (red) (right). Interaction with the 60S subunit is via the Zuotin homology domain (ZHD). Regions of Zuo1/Zuotin towards the C-terminus (boxed) are important for interaction with the 30S subunit; ribosomal protein ul22 (22) interacts both with helix 44 (h24) and the interior of the tunnel, serving as a potential site for monitoring the nascent chain. As discussed in the text, the architecture and function of Zuo1/Zuotin illustrate how some J-proteins have evolved complex activities; C-terminal SANT domains of human and the 13 C-terminal residues of Zuo1 (*) are both involved in cellular regulation. A second J-protein (Jjj1/DNAJC21) also interacts with eukaryotic 60S ribosome particles via its ZHD (bottom). Jjj1 serves as an example of the difficulties in rigorously categorizing J-proteins by their structure and/or client binding properties. While localized to its site of action, it also binds a specific substrate protein, Rei1, as discussed in the text. (B) J-protein functioning in protein translocation are integral membrane proteins and also interaction with other proteins of the translocation apparatus. In the case of the import motor of the mitochondrial inner membrane (right), Pam18/DNAJC19 interacts with translocon subunit Tim17 on the intermembrane space (IMS) side and indirectly on the matrix side (with the import motor component Pam16 directly; Pam16 directly interacts with Tim44, which directly interacts with the import channel). J-protein names from S. cerevisiae (Sc) and H. sapiens (Hs) are listed. Domain organization of J-proteins is drawn to scale except where indicated by hatch marks (//); J- J-domain; TM- transmembrane region.
Similar articles
- The HSP70 chaperone machinery: J proteins as drivers of functional specificity.
Kampinga HH, Craig EA. Kampinga HH, et al. Nat Rev Mol Cell Biol. 2010 Aug;11(8):579-92. doi: 10.1038/nrm2941. Nat Rev Mol Cell Biol. 2010. PMID: 20651708 Free PMC article. Review. - Not all J domains are created equal: implications for the specificity of Hsp40-Hsp70 interactions.
Hennessy F, Nicoll WS, Zimmermann R, Cheetham ME, Blatch GL. Hennessy F, et al. Protein Sci. 2005 Jul;14(7):1697-709. doi: 10.1110/ps.051406805. Protein Sci. 2005. PMID: 15987899 Free PMC article. Review. - Roles of intramolecular and intermolecular interactions in functional regulation of the Hsp70 J-protein co-chaperone Sis1.
Yu HY, Ziegelhoffer T, Osipiuk J, Ciesielski SJ, Baranowski M, Zhou M, Joachimiak A, Craig EA. Yu HY, et al. J Mol Biol. 2015 Apr 10;427(7):1632-43. doi: 10.1016/j.jmb.2015.02.007. Epub 2015 Feb 14. J Mol Biol. 2015. PMID: 25687964 Free PMC article. - Structural and Biochemical Properties of Hsp40/Hsp70 Chaperone System.
Faust O, Rosenzweig R. Faust O, et al. Adv Exp Med Biol. 2020;1243:3-20. doi: 10.1007/978-3-030-40204-4_1. Adv Exp Med Biol. 2020. PMID: 32297208 Review. - HSP40 proteins use class-specific regulation to drive HSP70 functional diversity.
Faust O, Abayev-Avraham M, Wentink AS, Maurer M, Nillegoda NB, London N, Bukau B, Rosenzweig R. Faust O, et al. Nature. 2020 Nov;587(7834):489-494. doi: 10.1038/s41586-020-2906-4. Epub 2020 Nov 11. Nature. 2020. PMID: 33177718
Cited by
- Pseudophosphorylation of single residues of the J-domain of DNAJA2 regulates the holding/folding balance of the Hsc70 system.
Velasco-Carneros L, Bernardo-Seisdedos G, Maréchal JD, Millet O, Moro F, Muga A. Velasco-Carneros L, et al. Protein Sci. 2024 Aug;33(8):e5105. doi: 10.1002/pro.5105. Protein Sci. 2024. PMID: 39012012 Free PMC article. - Chaperone Networks in Fungal Pathogens of Humans.
Horianopoulos LC, Kronstad JW. Horianopoulos LC, et al. J Fungi (Basel). 2021 Mar 12;7(3):209. doi: 10.3390/jof7030209. J Fungi (Basel). 2021. PMID: 33809191 Free PMC article. Review. - Take Me Home, Protein Roads: Structural Insights into Signal Peptide Interactions during ER Translocation.
Liaci AM, Förster F. Liaci AM, et al. Int J Mol Sci. 2021 Nov 1;22(21):11871. doi: 10.3390/ijms222111871. Int J Mol Sci. 2021. PMID: 34769302 Free PMC article. Review. - Hsp70 at the membrane: driving protein translocation.
Craig EA. Craig EA. BMC Biol. 2018 Jan 17;16(1):11. doi: 10.1186/s12915-017-0474-3. BMC Biol. 2018. PMID: 29343244 Free PMC article. Review. - Molecular chaperones: from proteostasis to pathogenesis.
Ravindran MS. Ravindran MS. FEBS J. 2018 Sep;285(18):3353-3361. doi: 10.1111/febs.14576. Epub 2018 Jun 22. FEBS J. 2018. PMID: 29890022 Free PMC article.
References
- Balchin D, et al. In vivo aspects of protein folding and quality control. Science. 2016;353(6294):aac4354. - PubMed
- Mayer MP. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 2013;38(10):507–14. - PubMed
- Fotin A, et al. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature. 2004;432(7017):649–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources