Bacterial secretion system skews the fate of Legionella-containing vacuoles towards LC3-associated phagocytosis - PubMed (original) (raw)
Bacterial secretion system skews the fate of Legionella-containing vacuoles towards LC3-associated phagocytosis
Andree Hubber et al. Sci Rep. 2017.
Abstract
The evolutionarily conserved processes of endosome-lysosome maturation and macroautophagy are established mechanisms that limit survival of intracellular bacteria. Similarly, another emerging mechanism is LC3-associated phagocytosis (LAP). Here we report that an intracellular vacuolar pathogen, Legionella dumoffii, is specifically targeted by LAP over classical endocytic maturation and macroautophagy pathways. Upon infection, the majority of L. dumoffii resides in ER-like vacuoles and replicate within this niche, which involves inhibition of classical endosomal maturation. The establishment of the replicative niche requires the bacterial Dot/Icm type IV secretion system (T4SS). Intriguingly, the remaining subset of L. dumoffii transiently acquires LC3 to L. dumoffii-containing vacuoles in a Dot/Icm T4SS-dependent manner. The LC3-decorated vacuoles are bound by an apparently undamaged single membrane, and fail to associate with the molecules implicated in selective autophagy, such as ubiquitin or adaptors. The process requires toll-like receptor 2, Rubicon, diacylglycerol signaling and downstream NADPH oxidases, whereas ULK1 kinase is dispensable. Together, we have discovered an intracellular pathogen, the survival of which in infected cells is limited predominantly by LAP. The results suggest that L. dumoffii is a valuable model organism for examining the mechanistic details of LAP, particularly induced by bacterial infection.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
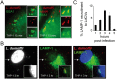
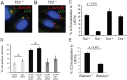
Figure 1. Localization of endosomal markers on LdCVs.
(A) _L. dumoffii_-containing vacuoles (LdCVs) preclude an early endosomal marker the early-endosomal antigen 1 (EEA1). Association of EEA1 with LdCVs was examined in THP-1 cells at 15 min post-infection. EEA1 was visualized by indirect immunofluorescence using the anti-EEA antibody (Sigma-Aldrich #E4156). Cells were infected with L. dumoffii strains constitutively expressing mCherry. (B) Some LdCVs acquire late endosomal and lysosomal marker LAMP-1 in THP-1 cells at 3 hours post infection (arrows and insets). LAMP-1 association with mCherry-expressing L. dumoffii was examined using indirect immunofluorescence with H4A3 (Santa Cruz #sc-20011). (C) Quantification of LAMP-1 recruitment to LdCVs at indicated time points post infection in THP-1 cells. Data is averaged values ± standard error of the mean (SEM) from two independent experiments, each performed in triplicate with 100 vacuoles scored for each sample.
Figure 2. LdCVs become transiently decorated with LC3 in a Dot/Icm-dependent manner.
(A) Confocal z-stack of RAW cells stably expressing GFP-LC3 after infection with L. dumoffii for 3 hours. The representative LC3-positive vacuole is shown by a white arrow. (B) Quantification of LC3-association to LdCVs and LpCVs over time in THP-1 cells. Wild-type L. dumoffii capable of translocating bacterial effectors elicited LC3-decoration, whereas the translocation-deficient strains (∆dotA) did not. (C) L. dumoffiii ∆flaA in a mouse embryonic fibroblast (MEF) at 16 hours post-infection. Despite the presence of LC3-positive puncta (white arrows), significant association with the bacterial compartment (dotted white square) was not observed. (D) Representative images of LC3-association with T4SS+ and T4SS− (∆dotA) L. dumoffii in Atg7+/− MEF cells, and T4SS+ L. dumoffii in _Atg7_−/− MEF cells. In panels C and D, LC3 was detected by indirect immunofluorescence using anti-LC3 antibody (MBL#PM036). Red-coloured L. dumoffii constitutively express mCherry.
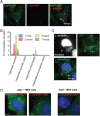
Figure 3. LdCVs become decorated neither with ubiquitin nor with selective-autophagy adaptor proteins.
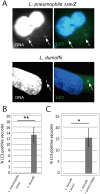
(A) Representative micrographs of THP-1 cells infected with L. dumoffii or L. pneumophila for 3 hours. Nuclear and bacterial DNAs are stained with Hoechst 33342, and ubiquitin is stained using anti-ubiquitin antibody (clone FK2). Arrows describe locations of bacteria. (B) Quantification of ubiquitin association with vacuoles containing either L. pneumophila or L. dumoffii in THP-1 cells. Data is averaged values ± standard error of the mean (SEM) from two independent experiments, each performed in triplicate with 100 vacuoles scored for each sample. (C) Representative micrograph of THP-1 cells co-infected with mCherry-expressing L. pneumophila and unlabelled L. dumoffii at 3 hours post-infection. Ubiquitin staining was performed using anti-ubiquitin (FK2) antibody. The blue (Hoescht 33342) but not red bacteria are L. dumoffii. Ubiquitin was commonly associated with LpCVs (arrows), but was absent from LdCVs (white dotted squares). (D,E) Fluorescent micrographs of RAW264.7 cells stably expressing GFP-LC3 and infected with L. dumoffii (D) or S. Typhimurium (E). Ubiquitin staining was performed using anti-ubiquitin (FK2) antibody. Both nuclear and bacterial DNAs are shown in blue (Hoescht 3342). (F) Quantification of ubiquitin association with GFP-LC3-positive LdCVs and SCVs in RAW264.7 cells at 3 hours post-infection. Data is average of two independent experiments performed in triplicate. (G) Quantification of localization of indicated autophagy adaptor proteins to LdCVs (grey bars) and to SCVs (black bars) in HeLa cells. Data is average from two independent experiments, each performed in triplicate. See Figure S2 for detailed experimental procedure.
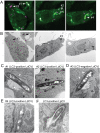
Figure 4. LC3-associated LdCV is bordered by a single membrane.
(A,B) CLEM analysis of LdCVs. RAW264.7 cells stably expressing EYGP-LC3 were cultured on glass bottom dishes (with grids) and then infected with mCherry-expressing L. dumoffii for 2.5–3 hours. Cells were fixed and fluorescent images obtained using a confocal microscope (A). Specimens were then further examined by transmission electron microscopy (TEM) (rest of all panels). TEM images are of the same field as the fluorescent micrographs (B). The representative LdCVs indicated by arrows with number (#1–#4) were further analysed by high-magnification TEM (C–E). (C) High-magnification TEM images of LC3-positive single-membrane-bound LdCVs. Small intracellular vesicles are denoted by white arrows (see text) in TEM images of LdCV #1. (D) A representative LC3-negative LdCV was also examined, as shown by LdCV #3. A large black arrow shows nearby mitochondria with internal cristae visible and a white arrow shows vesicles attached to the LdCV. (E) The LdCV #4 is a rare (~7%) LC3-positive double-membrane LdCVs, which is consistent with conventional autophagy. (F) A micrograph of a rare LC3-positive LpCVs formed in the RAW264.7 GFP-LC3 cell line.
Figure 5. LC3-recruitement to LdCVs is dependent on TLR2 but not Ulk1.
(A–C) Bone marrow-derived macrophages were obtained from the femur and tibia of heterozygous Tlr2+/− and Tlr4+/− mice and homozygous _Tlr2_−/− and Tlr4_−/− mice. After differentiation into bone-marrow derived macrophages, cells were infected with mCherry expressing L. dumoffii ∆_flaA for 3 hours. LC3 was detected by indirect immunofluorescence (A,B) and localization scored in three independent experiments (C). (D) Quantification of LC3-recruitment in mock, TLR2 or TLR4-expressing HEK293 cells treated with scrambled siRNA or Ulk1 siRNA for 3 days prior to infection with mCherry expressing L. dumoffii ∆flaA for 3 hours. Data are the average of three independent experiments, each performed with triplicate wells. (E) Bone marrow-derived macrophages were obtained from the femur and tibia of heterozygous Rubicon+/− and homozygous Rubicon−/− mice. L. dumoffii infection and LC3 detection was carried out as described for panels A–C.
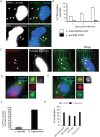
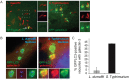
Figure 6. LC3-associated LdCVs preclude galectin-3.
(A) HeLa cells stably expressing FcγRII receptor were co-transfected with pTLR2 and either GFP-galectin-3. Images shown are at 2 hours post-infection for opsonized L. dumoffii (left) and S. Typhimurium (right), both organisms harbour plasmids for constitutive expression of mCherry (red). (B) Representative fluorescent micrographs showing co-localization of GFP-LC3 and galectin-3 on SCVs but not LdCVs. RAW264.7 cells stably expressing GFP-LC3 were infected with either S. Typhimurium (right) or L. dumoffii (left). Bacteria are shown in blue (Hoescht 3342). Galectin-3 was detected by indirect immunofluorescence using an anti-galectin-3 antibody (Santa Cruz #sc-23938). (C) Quantification of assay shown in panel B. Data are average of three independent experiments, each performed in triplicate.
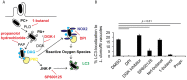
Figure 7. Host factors involved in LAP-targeting of LdCVs.
(A) Diagram showing proposed pathway of DAG formation on bacterial phagosomes using the host enzymes phospholipase D (PLD) and phosphatidic acid phosphatase (PAP). DAG is recognized by protein kinase C (PKC), which phosphorylates an essential component of NADPH oxidases and c-Jun N-terminal kinase (JNK). Pharmacological inhibitors are shown in red. (B) Quantification of single phagosome analysis of LC3-recruitment to _L. dumoffii_-containing vacuoles in THP-1 cells at 3 hours post-infection. Cells were treated with growth media containing DMSO, DPI (10 μM), DGK inhibitor R59 022 (10 μM), SP600125 (20 μM), tert-butanol (negative control), 1-butanol or propranolol hydrochloride (250 μM) at the point of infection and were maintained throughout the experiment. Data are average of three independent experiments, each performed in triplicate. In all relevant experiments, significance was determined using the two-tailed Student’s t-test.
Figure 8. LAP-targeting of LdCVs results in L. dumoffii degradation.
(A) Representative fluorescent image showing co-localization of GFP-LC3 and LAMP1 to an LdCV at 3 hours post-infection in RAW264.7 cells. LAMP1 was detected by indirect immunofluorescence using anti-LAMP1 (clone H4A3; Santa Cruz #sc-20011). Hoescht 33342 (blue) was used to stain both nuclear and bacterial DNA. (B) Images show dual antibody labelling of LC3 and LAMP1 on an LdCV at 3 hours post-infection in THP-1 cells. (C) Quantification at 3 hours showed 55% of LC3-positive vacuoles also possessed the lysosomal marker LAMP1 in THP-1 cells. However, ubiquitin is not a common feature of the LC3-positive L. dumoffii vacuole. (D) Atg7+/− and Atg7_−/− knockout MEF cells were infected with L. dumoffii ∆_flaA and intracellular survival was assessed by counting colony forming units at five hours post-infection. (E) Bone marrow-derived macrophages from Tlr2+/− and Tlr2_−/− mice were infected with L. dumoffii ∆_flaA and intracellular survival was assessed by counting colony-forming units at five hours post-infection. _P_-values determined by the two-tailed Student’s t-test are shown.
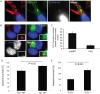
Figure 9. Effects of RavZ in LC3-recruitment to LpCVs and LdCVs.
(A) THP-1 cells were infected with a L. pneumophila strain lacking ravZ or a wild-type L. dumoffii which does not encode ravZ for 3 hours. LC3 was stained with LC3 antibodies. Bacteria were visualized with DAPI-staining (white arrows). (B) Quantification of LC3-association on Δ_ravZ_ LpCVs and LdCVs. (C) THP-1 cells were infected with a wild-type L. dumoffii having a pMMB207-derived plasmid expressing L. pneumophila ravZ or its catalytic mutant _ravZ_C258A for 3 hours. LC3-associated LdCVs were quantified as in (B). Significance of the results was determined using the two-tailed Student’s t-test; **p < 0.01, *p < 0.03.
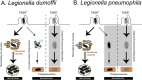
Figure 10. Proposed three alternative fates of intracellular Legionella.
L. dumoffii lacking ability to translocate effectors (without Dot/Icm T4SS) are cleared through endocytic maturation and trafficking to degradative lysosomes (shaded). However, the fate of virulent (with Dot/Icm T4SS) L. dumoffii is more complex. Though the majority (~80%) will subvert canonical endocytic maturation and go on to replicate inside ER-like compartments, some (~20%) will be degraded following initiation of LC3-associated phagocytosis (LAP). Thus, the nature and kinetics of unproductive L. dumoffii trafficking differ substantially from those of L. pneumophila.
Similar articles
- LAP-like non-canonical autophagy and evolution of endocytic vacuoles in pancreatic acinar cells.
De Faveri F, Chvanov M, Voronina S, Moore D, Pollock L, Haynes L, Awais M, Beckett AJ, Mayer U, Sutton R, Criddle DN, Prior IA, Wileman T, Tepikin AV. De Faveri F, et al. Autophagy. 2020 Jul;16(7):1314-1331. doi: 10.1080/15548627.2019.1679514. Epub 2019 Oct 25. Autophagy. 2020. PMID: 31651224 Free PMC article. - The autophagic response to Staphylococcus aureus provides an intracellular niche in neutrophils.
Prajsnar TK, Serba JJ, Dekker BM, Gibson JF, Masud S, Fleming A, Johnston SA, Renshaw SA, Meijer AH. Prajsnar TK, et al. Autophagy. 2021 Apr;17(4):888-902. doi: 10.1080/15548627.2020.1739443. Epub 2020 Mar 15. Autophagy. 2021. PMID: 32174246 Free PMC article. - Legionella RavZ Plays a Role in Preventing Ubiquitin Recruitment to Bacteria-Containing Vacuoles.
Kubori T, Bui XT, Hubber A, Nagai H. Kubori T, et al. Front Cell Infect Microbiol. 2017 Aug 28;7:384. doi: 10.3389/fcimb.2017.00384. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28971069 Free PMC article. - LC3-associated phagocytosis in microbial pathogenesis.
Schille S, Crauwels P, Bohn R, Bagola K, Walther P, van Zandbergen G. Schille S, et al. Int J Med Microbiol. 2018 Jan;308(1):228-236. doi: 10.1016/j.ijmm.2017.10.014. Epub 2017 Nov 21. Int J Med Microbiol. 2018. PMID: 29169848 Review. - Formation of the Legionella Replicative Compartment at the Crossroads of Retrograde Trafficking.
Bärlocher K, Welin A, Hilbi H. Bärlocher K, et al. Front Cell Infect Microbiol. 2017 Nov 24;7:482. doi: 10.3389/fcimb.2017.00482. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29226112 Free PMC article. Review.
Cited by
- Bacterial Manipulation of Autophagic Responses in Infection and Inflammation.
Jiao Y, Sun J. Jiao Y, et al. Front Immunol. 2019 Dec 3;10:2821. doi: 10.3389/fimmu.2019.02821. eCollection 2019. Front Immunol. 2019. PMID: 31849988 Free PMC article. Review. - Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy.
Mitchell G, Cheng MI, Chen C, Nguyen BN, Whiteley AT, Kianian S, Cox JS, Green DR, McDonald KL, Portnoy DA. Mitchell G, et al. Proc Natl Acad Sci U S A. 2018 Jan 9;115(2):E210-E217. doi: 10.1073/pnas.1716055115. Epub 2017 Dec 26. Proc Natl Acad Sci U S A. 2018. PMID: 29279409 Free PMC article. - LAP it up, fuzz ball: a short history of LC3-associated phagocytosis.
Martinez J. Martinez J. Curr Opin Immunol. 2018 Dec;55:54-61. doi: 10.1016/j.coi.2018.09.011. Epub 2018 Oct 2. Curr Opin Immunol. 2018. PMID: 30286399 Free PMC article. Review. - LAP-like non-canonical autophagy and evolution of endocytic vacuoles in pancreatic acinar cells.
De Faveri F, Chvanov M, Voronina S, Moore D, Pollock L, Haynes L, Awais M, Beckett AJ, Mayer U, Sutton R, Criddle DN, Prior IA, Wileman T, Tepikin AV. De Faveri F, et al. Autophagy. 2020 Jul;16(7):1314-1331. doi: 10.1080/15548627.2019.1679514. Epub 2019 Oct 25. Autophagy. 2020. PMID: 31651224 Free PMC article. - Extracellular vesicles deliver Mycobacterium RNA to promote host immunity and bacterial killing.
Cheng Y, Schorey JS. Cheng Y, et al. EMBO Rep. 2019 Mar;20(3):e46613. doi: 10.15252/embr.201846613. Epub 2019 Jan 25. EMBO Rep. 2019. PMID: 30683680 Free PMC article.
References
- Escoll P., Rolando M., Gomez-Valero L. & Buchrieser C. From amoeba to macrophages: exploring the molecular mechanisms of Legionella pneumophila infection in both hosts. Curr Top Microbiol Immunol 376, 1–34 (2013). - PubMed
- Berger K. H. & Isberg R. R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol 7, 7–19 (1993). - PubMed
- Brand B. C., Sadosky A. B. & Shuman H. A. The Legionella pneumophila icm locus: a set of genes required for intracellular multiplication in human macrophages. Mol Microbiol 14, 797–808 (1994). - PubMed
- Hubber A. & Roy C. R. Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26, 261–283 (2010). - PubMed
- Diederen B. M. Legionella spp. and Legionnaires’ disease. J Infect 56, 1–12 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials