Ubiquitylation of p62/sequestosome1 activates its autophagy receptor function and controls selective autophagy upon ubiquitin stress - PubMed (original) (raw)
. 2017 May;27(5):657-674.
doi: 10.1038/cr.2017.40. Epub 2017 Mar 21.
Hong Peng 1 2 3, Jiao Yang 1 2 3, Qing You 1 2, Wen Han 1, Tianrang Li 1, Daming Gao 1, Xiaoduo Xie 1, Byung-Hoon Lee 4, Juan Du 5, Jian Hou 5, Tao Zhang 6, Hai Rao 7, Ying Huang 3, Qinrun Li 1, Rong Zeng 1, Lijian Hui 3, Hongyan Wang 1, Qin Xia 8, Xuemin Zhang 8, Yongning He 3, Masaaki Komatsu 9, Ivan Dikic 10, Daniel Finley 4, Ronggui Hu 1
Affiliations
- PMID: 28322253
- PMCID: PMC5520855
- DOI: 10.1038/cr.2017.40
Ubiquitylation of p62/sequestosome1 activates its autophagy receptor function and controls selective autophagy upon ubiquitin stress
Hong Peng et al. Cell Res. 2017 May.
Abstract
Alterations in cellular ubiquitin (Ub) homeostasis, known as Ub stress, feature and affect cellular responses in multiple conditions, yet the underlying mechanisms are incompletely understood. Here we report that autophagy receptor p62/sequestosome-1 interacts with E2 Ub conjugating enzymes, UBE2D2 and UBE2D3. Endogenous p62 undergoes E2-dependent ubiquitylation during upregulation of Ub homeostasis, a condition termed as Ub+ stress, that is intrinsic to Ub overexpression, heat shock or prolonged proteasomal inhibition by bortezomib, a chemotherapeutic drug. Ubiquitylation of p62 disrupts dimerization of the UBA domain of p62, liberating its ability to recognize polyubiquitylated cargoes for selective autophagy. We further demonstrate that this mechanism might be critical for autophagy activation upon Ub+ stress conditions. Delineation of the mechanism and regulatory roles of p62 in sensing Ub stress and controlling selective autophagy could help to understand and modulate cellular responses to a variety of endogenous and environmental challenges, potentially opening a new avenue for the development of therapeutic strategies against autophagy-related maladies.
Figures
Figure 1
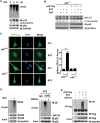
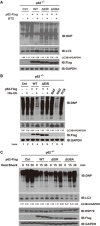
Autophagy receptor p62 is ubiquitylated upon autophagy activation induced by prolonged proteasome inhibition. (A) Autophagy was activated upon proteasomal inhibition in HeLa cells treated with bortezomib (BTZ, 1 μM for 6 or 12 h). (B) p62 was required for autophagy activated by BTZ. p62−/− MEF cells stably expressing wild-type human p62 treated with BTZ (1 μM for 12 h) or bafilomycin-A1 (BAF, 200 nM for 8 h) with the indicated combinations. Cells were lysed and analysed with indicated antibodies. The levels of lipidated LC3 were quantitated after normalization of that in each control as 1.0. (C) p62 was required for autophagy activated by BTZ. Puncta formation by GFP-LC3 was promoted in p62+/+ but not p62−/− MEF cells. Scale bar, 10 μm. (D, E) Endogenous or Flag-tagged p62 was ubiquitylated upon proteasome inhibition by BTZ (1 μM for 12 h) in HeLa (D) or HEK293T cells (E). Lysates were immunoprecipitated in denaturing RIPA buffer with indicated antibodies. Immunoprecipitates were treated with or without Ub-specific protease 2 (USP2cc), followed by immunoblotting analysis with indicated antibodies.
Figure 2
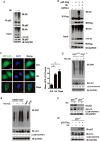
Overexpression of Ub alone activates autophagy in a p62-dependent manner. (A, B) Endogenous or Flag-tagged p62 was ubiquitylated upon Ub+ stress induced by Ub overexpression in HeLa cells (A) or HEK293T cells (B). Lysates were immuno-precipitated in denaturing RIPA buffer with indicated antibodies. Immunoprecipitates were treated with or without Ub-specific protease 2 (USP2cc), followed by immunoblotting analysis with indicated antibodies. (C) Puncta formation by GFP-LC3 was promoted in HeLa cells overexpressing Ub (∼ 500 pmol/mg total proteins). Scale bar, 10 μm. (D) Autophagy might be the main route to proteolytically remove cellular oxidatively damaged (carbonylated) proteins. ATG7+/+ or ATG7−/− MEF cells were transfected with vector or His-Ub, cultured for 24 h and treated with DMSO or bafilomycin-A1 (BAF, 200 nM for 8 h). Carbonylated proteins were visualized through derivatization with DNPH, followed by IB with anti-DNP. The levels of lipidated LC3 were quantitated, after normalization of that in each control as 1.0. (E) Autophagy was activated upon Ub+ stress induced by overexpression of Ub. HEK293T cells were transfected with vector or His-Ub, cultured for 24 h and treated with DMSO or BAF (200 nM for 8 h). Pyocyanin (PCN) was used as a positive control, and N-acetyl-ℒ-cysteine (NAC) was used as a negative control. Carbonylated proteins were visualized through derivatization with DNPH, followed by IB with anti-DNP. (F, G) p62 was required for autophagy activated by Ub+ stress. (F) p62+/+ or p62−/− MEF cells were subjected to Ub+ stress induced by overexpression of Ub. (G) p62−/− MEF cells with or without re-introduction of Flag-tagged human p62. The levels of lipidated LC3 were quantitated, after normalization of the signal in each control as 1.0.
Figure 3
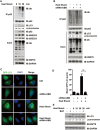
Heat shock also activates autophagy in dependence of p62. Endogenous human p62 underwent ubiquitylation upon autophagy activation during Ub+ stress induced by heat shock. HEK293FT cells transfected with or without siRNA-UBB, were subjected to heat shock (42 °C, 30 min or indicated times) prior to harvest, lysed in RIPA buffer, and followed by immunoblotting analysis with indicated antibodies, HSP70 used here as an indication of heat-shock treatment (A, B, E). (E) About 36 h after transfection, cells were treated with DMSO or bafilomycin (BAF, 200 nM, 8 h). The levels of lipidated LC3 were quantitated, after normalization of that in each control as 1.0. Puncta formation by GFP-LC3 in HeLa cells in indicated groups was visualized (C) and quantitated (D). Scale bar, 10 μm.
Figure 4
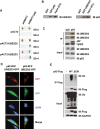
Human E2 conjugating enzymes, UBE2D2 or UBE2D3, interact with autophagy receptor p62 and support ubiquitylation of p62 in vitro and in vivo. (A) Human p62 interacted with UBE2D2 and UBE2D3 in yeast two hybrid assay. Co-transformation of MAV203 yeast strain with p62 and UBE2D2 or UBE2D3 supported survival of the cells on SD-4. (B) Human p62 directly interacted with UBE2D2 and UBE2D3 in GST pull-down assays carried out with purified GST-tagged p62 and His-tagged UBE2D2, or His-tagged p62 and GST-tagged UBE2D3. (C) Endogenous p62 protein formed complex with UBE2D2 and UBE2D3 in vivo. IgG and p62 antibodies were used to perform immunoprecipitation in HeLa cell lysates separately, then the precipitatants were subjected to immunoblotting analysis with anti-UBE2D2 or anti-UBE2D3. (D) Human p62 co-localized with UBE2D2 or UBE2D3. HeLa cells overexpressing p62-red fluorescent protein (RFP) and UBE2D2-GFP or UBE2D3-GFP were subjected to fluorescent microscopy analysis. Scale bar, 10 μm. (E) Upon Ub+ stress induced by overexpression of Ub, p62 underwent ubiquitylation in dependence of the interaction between p62 and UBE2D2 or UBE2D3. Human p62 interacted with UBE2D2 or UBE2D3 at E2-interacting region (EIR, spanning 294-320 residues). HeLa cells were co-transfected with His-ub and p62WT (wild-type human p62) or p62ΔEIR (the EIR deletion mutant of p62), and lysed in RIPA buffer 36 h after transfection. Immunoprecipitates were then subjected to immunoblotting analysis with indicated antibodies. SD-2, deficient in Trp, Leu; SD-4, deficient in Trp, Leu, Ura and His.
Figure 5
UBE2D2/UBE2D3-p62 interaction is critical for Ub+ stress-activated autophagy in vivo. (A-C) p62−/− MEF cells stably expressing p62WT (wild-type human p62), p62ΔEIR (the EIR deletion mutant of p62), or p62ΔUBA (the UBA deletion mutant of p62) were subjected to bortezomib treatment (BTZ, 1 μM for 12 h), Ub overexpression or heat shock (42 °C, 0, 15 or 30 min) before harvest. The residual cellular carbonylated proteins were visualized through derivatization with DNPH, followed by IB with anti-DNP, HSP70 used here as an indication of heat-shock treatment. The levels of lipidated LC3 were quantitated, after normalization of that in each control as 1.0.
Figure 6
UBE2D2- and UBE2D3-mediated ubiquitylation of p62 is required for the activation of autophagy upon Ub+ stress. (A-C) Upon Ub+ stress, endogenous p62 underwent ubiquitylation in dependence of UBE2D2 and UBE2D3. Wild-type or UBE2D2/UBE2D3 double knockout (KO) HEK293T cells were treated with proteasome inhibitor bortezomib (BTZ, 1 μM for 12 h) or transfected with vector or no-tagged Ub, or under heat-shock treatment and lysed in RIPA buffer. Endogenous p62 proteins were immunoprecipitated and subjected to IB using anti-p62 or anti-Ub, respectively. (D-F) UBE2D2- and UBE2D3-mediated ubiquitylation of p62 was required for the activation of autophagy upon Ub+ stress. Wild-type or UBE2D2/UBE2D3 double KO HEK293FT cells were treated with proteasome inhibitor BTZ (1 μM for 12 h), or transfected with vector or no-tagged Ub or under heat-shock treatment, after these treatments, cells were treated with DMSO or bafilomycin (BAF, 200 nM, 8 h), HSP70 used here as an indication of heat-shock treatment. The levels of lipidated LC3 were quantitated, after normalization of that in each control as 1.0.
Figure 7
Ubiquitylation of p62 switches on its recognition of poly-Ub chains, potentially through disrupting the formation of dimerization of the C-terminal UBA domains. (A) UBE2D2/UBE2D3 supported ubiquitylation of p62 in vitro on at least nine lysine residues (See Supplementary information, Figure S5 for details). All potential ubiquitylation sites in p62 were highlighted in purple. (B, C) Crystal structure of the p62 UBA dimer (PDB 3B0F). (B) Each monomer is colored in green or cyan. Lys420 and Glu409 on each monomer are shown as sticks, with the hydrogen bonds are shown as dash lines. (C) Ribbon diagram and surface representation of the p62 UBA domain dimer. Residues involved in ubiquitin binding are colored in red. (D, E) UBE2D2-/UBE2D3-supported ubiquitylation of p62 promoted the binding of the UBA domain to poly-Ub chains. Flag-tagged wild-type human p62 (WT) or mutants for polyubiquitylation (9KR), oligomerization deficiency (K7A/D69A), polyubiquitin binding deficiency mutant (F406V), phosphorylation deficiency mutant (S349A/S403A) and phosphorylation-mimicking mutant (S349E/S403E) were subjected to in vitro ubiquitylation reaction, unmodified or ubiquitylated p62 proteins enriched by anti-Flag M2 beads were incubated with pre-assembled HA-tagged poly-Ub chains. (F) Phosphorylation status on either Ser349 or Ser403 of p62 barely changed upon autophagy induced by Ub overexpression in HEK293T cells. Lysates were immunoprecipitated with anti-Flag beads in denaturing RIPA buffer, and followed by IB with anti-p-p62 (S403) or anti-p-p62 (S349). (G) UBE2D2-/UBE2D3-supported ubiquitylation of p62 promoted its binding to Ub conjugates, enriched from HEK293T cells transiently expressing HA-tagged Ub.
Figure 8
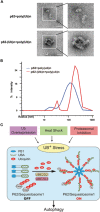
Visualization of the effect of p62 ubiquitylation on Ub binding. Negative stain electron microscopy (EM) (A) and dynamic light scattering (DLS) analyses (B) were performed to visualize and compare the complex formed by pre-assembled poly-Ub chains and unmodified or ubiquitylated p62 proteins. Scale bar: 50 nm. (C) A model depicting the “sensor” role of p62 in activating autophagy upon Ub+ stress induced by heat shock, Ub overexpression or proteasomal inhibition.
Comment in
- Love laughs at Locksmiths: Ubiquitylation of p62 unlocks its autophagy receptor potential.
Conway O, Kirkin V. Conway O, et al. Cell Res. 2017 May;27(5):595-597. doi: 10.1038/cr.2017.56. Epub 2017 Apr 21. Cell Res. 2017. PMID: 28429767 Free PMC article. - SQSTM1/p62 (sequestosome 1) senses cellular ubiquitin stress through E2-mediated ubiquitination.
Yang J, Peng H, Xu Y, Xie X, Hu R. Yang J, et al. Autophagy. 2018;14(6):1072-1073. doi: 10.1080/15548627.2017.1332566. Epub 2017 Nov 30. Autophagy. 2018. PMID: 28614034 Free PMC article.
Similar articles
- SQSTM1/p62 (sequestosome 1) senses cellular ubiquitin stress through E2-mediated ubiquitination.
Yang J, Peng H, Xu Y, Xie X, Hu R. Yang J, et al. Autophagy. 2018;14(6):1072-1073. doi: 10.1080/15548627.2017.1332566. Epub 2017 Nov 30. Autophagy. 2018. PMID: 28614034 Free PMC article. - Inhibition of Protein Ubiquitination by Paraquat and 1-Methyl-4-Phenylpyridinium Impairs Ubiquitin-Dependent Protein Degradation Pathways.
Navarro-Yepes J, Anandhan A, Bradley E, Bohovych I, Yarabe B, de Jong A, Ovaa H, Zhou Y, Khalimonchuk O, Quintanilla-Vega B, Franco R. Navarro-Yepes J, et al. Mol Neurobiol. 2016 Oct;53(8):5229-51. doi: 10.1007/s12035-015-9414-9. Epub 2015 Sep 26. Mol Neurobiol. 2016. PMID: 26409479 Free PMC article. - UBE2D3 contributes to myocardial ischemia-reperfusion injury by regulating autophagy in dependence of p62/SQSTM1.
Wang X, Yang P, Jiang Y, Xu Y, Wang N, Rao P, Yang L, Sun L, Lu D. Wang X, et al. Cell Signal. 2021 Nov;87:110118. doi: 10.1016/j.cellsig.2021.110118. Epub 2021 Aug 12. Cell Signal. 2021. PMID: 34391873 - The exploitation of host autophagy and ubiquitin machinery by Mycobacterium tuberculosis in shaping immune responses and host defense during infection.
Shariq M, Quadir N, Alam A, Zarin S, Sheikh JA, Sharma N, Samal J, Ahmad U, Kumari I, Hasnain SE, Ehtesham NZ. Shariq M, et al. Autophagy. 2023 Jan;19(1):3-23. doi: 10.1080/15548627.2021.2021495. Epub 2022 Jan 9. Autophagy. 2023. PMID: 35000542 Free PMC article. Review. - Proteasomal and Autophagic Degradation Systems.
Dikic I. Dikic I. Annu Rev Biochem. 2017 Jun 20;86:193-224. doi: 10.1146/annurev-biochem-061516-044908. Epub 2017 May 1. Annu Rev Biochem. 2017. PMID: 28460188 Review.
Cited by
- SQSTM1/p62 Promotes Cell Growth and Triggers Autophagy in Papillary Thyroid Cancer by Regulating the AKT/AMPK/mTOR Signaling Pathway.
Yu F, Ma R, Liu C, Zhang L, Feng K, Wang M, Yin D. Yu F, et al. Front Oncol. 2021 Apr 15;11:638701. doi: 10.3389/fonc.2021.638701. eCollection 2021. Front Oncol. 2021. PMID: 33937040 Free PMC article. - The Multifunctional Protein p62 and Its Mechanistic Roles in Cancers.
Ning S, Wang L. Ning S, et al. Curr Cancer Drug Targets. 2019;19(6):468-478. doi: 10.2174/1568009618666181016164920. Curr Cancer Drug Targets. 2019. PMID: 30332964 Free PMC article. Review. - Characterization of MORN2 stability and regulatory function in LC3-associated phagocytosis in macrophages.
Morita M, Kajiye M, Sakurai C, Kubo S, Takahashi M, Kinoshita D, Hori N, Hatsuzawa K. Morita M, et al. Biol Open. 2020 Jun 23;9(6):bio051029. doi: 10.1242/bio.051029. Biol Open. 2020. PMID: 32414768 Free PMC article. - Love laughs at Locksmiths: Ubiquitylation of p62 unlocks its autophagy receptor potential.
Conway O, Kirkin V. Conway O, et al. Cell Res. 2017 May;27(5):595-597. doi: 10.1038/cr.2017.56. Epub 2017 Apr 21. Cell Res. 2017. PMID: 28429767 Free PMC article. - Induced TRIM21 ISGylation by IFN-β enhances p62 ubiquitination to prevent its autophagosome targeting.
Jin J, Meng X, Huo Y, Deng H. Jin J, et al. Cell Death Dis. 2021 Jul 13;12(7):697. doi: 10.1038/s41419-021-03989-x. Cell Death Dis. 2021. PMID: 34257278 Free PMC article.
References
- Hershko A, Ciechanover A, Varshavsky A. Basic medical research award. The ubiquitin system. Nat Med 2000; 6:1073–1081. - PubMed
- Finley D, Ciechanover A, Varshavsky A. Ubiquitin as a central cellular regulator. Cell 2004; 116:S29–S32, 2p following S32. - PubMed
- Hanna J, Meides A, Zhang DP, Finley D. A ubiquitin stress response induces altered proteasome composition. Cell 2007; 129:747–759. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources