Optimising reliability of mouse performance in behavioural testing: the major role of non-aversive handling - PubMed (original) (raw)
Optimising reliability of mouse performance in behavioural testing: the major role of non-aversive handling
Kelly Gouveia et al. Sci Rep. 2017.
Abstract
Handling laboratory animals during test procedures is an important source of stress that may impair reliability of test responses. Picking up mice by the tail is aversive, stimulating stress and anxiety. Responses among anxious animals can be confounded further by neophobia towards novel test environments and avoidance of test stimuli in open areas. However, handling stress can be reduced substantially by using a handling tunnel, or cupping mice without restraint on the open hand. Here we establish whether non-aversive handling, brief prior familiarisation with the test arena and alternative stimulus placement could significantly improve performance of mice in behavioural tests. We use a simple habituation-dishabituation paradigm in which animals must discriminate between two urine stimuli in successive trials, a task that mice can easily perform. Tail handled mice showed little willingness to explore and investigate test stimuli, leading to poor test performance that was only slightly improved by prior familiarisation. By contrast, those handled by tunnel explored readily and showed robust responses to test stimuli regardless of prior familiarisation or stimulus location, though responses were more variable for cup handling. Our study shows that non-aversive tunnel handling can substantially improve mouse performance in behavioural tests compared to traditional tail handling.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Figure 1. The habituation-dishabituation task.
(a) Principle underlying the habituation-dishabituation task. Repeated presentation of a novel stimulus for three successive trials (light blue bars) induces a reduction in investigation as the animal recognises an increasingly familiar stimulus (habituation, measured as less investigation in trial 3 compared to trial 1). When an unfamiliar stimulus is presented on the fourth trial (dark blue bar), investigation increases if the animal recognises that the stimulus is novel (dishabituation, measured as more investigation in trial 4 than trial 3). (b) Test arena, showing the location of a urine stimulus either at the centre (cr) or periphery (pr) of the arena. Mice were introduced at the lower edge of the arena (start location marked with X). Animals handled with a tunnel were tipped out backwards at floor level. Urine stimuli used in trials 1–4 came from unrelated male mice.
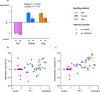
Figure 2. Handling method and stimulus location influences test performance in a habituation-dishabituation task.
In experiment 1, time sniffing a urine stimulus was recorded in successive trials (1–4), with urine derived from one male in trials 1–3 and from a different male in trial 4. Mice were handled by tail (pink), tunnel familiar from the home cage (blue) or cupped on the hand (orange). Data are medians ± interquartile range. (a) Wilcoxon signed ranks tests assessed habituation (reduced stimulus investigation of the same scent in trial 3 compared to trial 1), and dishabituation (greater investigation of novel scent in trial 4 compared to familiar scent in trial 3). Tests with the stimulus in different locations are pooled. Comparison of (b) habituation responses (trial 1-trial 3 investigation) and (c) dishabituation responses (trial 4-trial 3 investigation) according to handling method and stimulus location (arena centre: cr, solid bars; periphery: pr, hatched bars). P values from likelihood ratio tests comparing mixed effects models (Table 1). N sizes: Tail cr (8), pr (8); Tunnel cr (8), pr (7); Cup cr (6), pr (7).
Figure 3. Effects of handling method and stimulus location on exploratory behaviour in experiment 1.
PCA extracted a single component (PC1) that accounted for 75% of variance in behaviour averaged over four successive trials for each subject. This contrasted high positive weights for active exploration (movement around the arena: 0.94, visits to the stimulus: 0.92, time sniffing stimulus: 0.83) with negative weighting for cautious behaviour (frequency of stretched attend postures: −0.77). (a) Effects of handling method and stimulus location on PC1 scores (means ± sem), P values from likelihood ratio tests comparing mixed effects models (Table 1). Correlation between exploratory behaviour and (b) habituation (stimulus investigation in trial 1-trial 3), or (c) dishabituation (stimulus investigation in trial 4-trial 3). The stimulus was placed in the centre (squares) or periphery (triangles) of the arena and animals were handled by tail (pink), tunnel familiar from the home cage (blue) or cupped on the open hand (orange). N sizes as in Fig. 2.
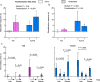
Figure 4. Familiarisation and handling method influence test performance in the habituation-dishabituation task.
Comparison of (a) habituation (trial 1-trial 3 stimulus investigation) and (b) dishabituation (trial 4-trial 3 investigation) according to handling method (pink: tail; blue: tunnel) and prior familiarisation (familiarised: f, cross hatched; naïve: n, solid bars). Data are medians ± interquartile range, P values from likelihood ratio tests comparing mixed effects models (Table 2). (c) Time sniffing the urine stimulus in successive trials (1–4) for mice handled by tail (pink) or tunnel (blue) when naïve (solid bars) or familiarised (cross hatched bars) with the test arena (medians ± interquartile range). P values from Wilcoxon matched pairs exact tests examining habituation (greater stimulus investigation of same scent in trial 1 than trial 3), and dishabituation (greater investigation of novel scent in trial 4 compared to familiar scent in trial 3). N = 8 mice in each method x familiarisation group.
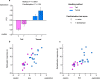
Figure 5. Effects of familiarisation and handling method on exploratory behaviour in experiment 2.
PCA extracted a single component (PC1) that accounted for 78% of variance in behaviour averaged over four successive trials. This contrasted high positive weights for active exploration (movement around the arena: 0.95, visits to the stimulus: 0.95, time sniffing stimulus: 0.92) with negative weighting for cautious behaviour (frequency of stretched attend postures: −0.67). (a) PC1 scores (means ± s.e.m.) according to handling method and familiarisation (familiarised: f, cross hatched; naïve: n, solid bars). P values from likelihood ratio tests comparing mixed effects models (Table 2). Correlation between exploratory behaviour and (b) habituation (stimulus investigation in trial 1–3), or (c) dishabituation (stimulus investigation in trial 4–3). Mice were either naïve (squares) or familiarised with the test arena (circles) prior to testing, and handled by the tail (pink), or with a tunnel familiar from the home cage (blue). N = 8 in each method x familiarisation group.
Similar articles
- Improving the practicality of using non-aversive handling methods to reduce background stress and anxiety in laboratory mice.
Gouveia K, Hurst JL. Gouveia K, et al. Sci Rep. 2019 Dec 30;9(1):20305. doi: 10.1038/s41598-019-56860-7. Sci Rep. 2019. PMID: 31889107 Free PMC article. - Benefits of tunnel handling persist after repeated restraint, injection and anaesthesia.
Henderson LJ, Dani B, Serrano EMN, Smulders TV, Roughan JV. Henderson LJ, et al. Sci Rep. 2020 Sep 3;10(1):14562. doi: 10.1038/s41598-020-71476-y. Sci Rep. 2020. PMID: 32884048 Free PMC article. - Identifying obstacles preventing the uptake of tunnel handling methods for laboratory mice: An international thematic survey.
Henderson LJ, Smulders TV, Roughan JV. Henderson LJ, et al. PLoS One. 2020 Apr 14;15(4):e0231454. doi: 10.1371/journal.pone.0231454. eCollection 2020. PLoS One. 2020. PMID: 32287297 Free PMC article. - Non-aversive handling in laboratory animals and its effects on depressive-like and anxiety-related behaviors: A scoping review.
Castro de Jesus L, S Rodrigues AL. Castro de Jesus L, et al. Physiol Behav. 2025 May 15;294:114883. doi: 10.1016/j.physbeh.2025.114883. Epub 2025 Mar 15. Physiol Behav. 2025. PMID: 40096937 - Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion.
Blanchard DC, Griebel G, Blanchard RJ. Blanchard DC, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2003 Dec;27(8):1177-85. doi: 10.1016/j.pnpbp.2003.09.012. Prog Neuropsychopharmacol Biol Psychiatry. 2003. PMID: 14659473 Review.
Cited by
- Influence of Diet on Reproducible Corticosterone Levels in a Mouse Model of Maternal Separation with Early Weaning.
Choe JY, Donkor M, Thorpe RJ Jr, Allen MS, Phillips NR, Jones HP. Choe JY, et al. Life (Basel). 2024 Jul 15;14(7):880. doi: 10.3390/life14070880. Life (Basel). 2024. PMID: 39063633 Free PMC article. - A Guide to the Generation of a 6-Hydroxydopamine Mouse Model of Parkinson's Disease for the Study of Non-Motor Symptoms.
Masini D, Plewnia C, Bertho M, Scalbert N, Caggiano V, Fisone G. Masini D, et al. Biomedicines. 2021 May 25;9(6):598. doi: 10.3390/biomedicines9060598. Biomedicines. 2021. PMID: 34070345 Free PMC article. - Post Mortem Study on the Effects of Routine Handling and Manipulation of Laboratory Mice.
Assenmacher CA, Lanza M, Tarrant JC, Gardiner KL, Blankemeyer E, Radaelli E. Assenmacher CA, et al. Animals (Basel). 2022 Nov 22;12(23):3234. doi: 10.3390/ani12233234. Animals (Basel). 2022. PMID: 36496755 Free PMC article. - Repeatability analysis improves the reliability of behavioral data.
Rudeck J, Vogl S, Banneke S, Schönfelder G, Lewejohann L. Rudeck J, et al. PLoS One. 2020 Apr 2;15(4):e0230900. doi: 10.1371/journal.pone.0230900. eCollection 2020. PLoS One. 2020. PMID: 32240211 Free PMC article. - Refinements to rodent head fixation and fluid/food control for neuroscience.
Barkus C, Bergmann C, Branco T, Carandini M, Chadderton PT, Galiñanes GL, Gilmour G, Huber D, Huxter JR, Khan AG, King AJ, Maravall M, O'Mahony T, Ragan CI, Robinson ESJ, Schaefer AT, Schultz SR, Sengpiel F, Prescott MJ. Barkus C, et al. J Neurosci Methods. 2022 Nov 1;381:109705. doi: 10.1016/j.jneumeth.2022.109705. Epub 2022 Sep 9. J Neurosci Methods. 2022. PMID: 36096238 Free PMC article. Review.
References
- Wahlsten D. In Mouse Behavioral Testing: How to Use Mice in Behavioral Neuroscience (ed.Wahlsten D.) 177–201 (Academic Press, 2011).
- Bailey K. R., Rustay N. R. & Crawley J. N. Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J 47, 124–131 (2006). - PubMed
- Schellinck H. How many ways can mouse behavioral experiments go wrong? Confounding variables in mouse models of neurodegenerative diseases and how to control them. Advances in the Study of Behavior 41, 255–366 (2010).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources