Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? - PubMed (original) (raw)
Review
Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link?
Viktor I Korolchuk et al. EBioMedicine. 2017 Jul.
Abstract
Cell senescence is increasingly recognized as a major contributor to the loss of health and fitness associated with aging. Senescent cells accumulate dysfunctional mitochondria; oxidative phosphorylation efficiency is decreased and reactive oxygen species production is increased. In this review we will discuss how the turnover of mitochondria (a term referred to as mitophagy) is perturbed in senescence contributing to mitochondrial accumulation and Senescence-Associated Mitochondrial Dysfunction (SAMD). We will further explore the subsequent cellular consequences; in particular SAMD appears to be necessary for at least part of the specific Senescence-Associated Secretory Phenotype (SASP) and may be responsible for tissue-level metabolic dysfunction that is associated with aging and obesity. Understanding the complex interplay between these major senescence-associated phenotypes will help to select and improve interventions that prolong healthy life in humans.
Search strategy and selection criteria: Data for this review were identified by searches of MEDLINE, PubMed, and references from relevant articles using the search terms "mitochondria AND senescence", "(autophagy OR mitophagy) AND senescence", "mitophagy AND aging" and related terms. Additionally, searches were performed based on investigator names. Abstracts and reports from meetings were excluded. Articles published in English between 1995 and 2017 were included. Articles were selected according to their relevance to the topic as perceived by the authors.
Keywords: Aging; Mitochondria; Mitophagy; Senescence.
Copyright © 2017 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures
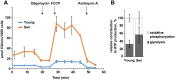
Fig. 1
Senescence drives changes in cell bioenergetics. Human MRC5 fibroblasts were either fully proliferation competent (young) or replicatively senescent (sen). Cellular oxygen consumption rates and extracellular acidification rates were assayed in medium containing 5 mM glucose, 2 mM
l
-glutamate and 3% FBS using a Seahorse XF24 analyzer. A) Oxygen consumption rates showing basal condition, and after sequential additions of the following drugs as indicated: oligomycin (to block ATP synthesis), the uncoupler FCCP (to stimulate respiration) and Antimycin A (to block the electron transport chain at complex III). B) Relative glycolytic and mitochondrial ATP production rates were calculated using the data generated in Fig. 1A as described (Mookerjee and Brand, 2015). Data are mean ± SD from 20 samples from 2 independent experiments.
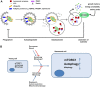
Fig. 2
The potential role of mitophagy in senescence. A) Diagram showing the process of autophagy, exemplified by mitophagy. Cytoplasmic contents including organelles such as mitochondria are sequestered into double-membraned phagophore structures. This process can be selective and involves a number of receptor proteins; specifically for mitochondria these include NDP52, optineurin and TAXBP1. These receptor proteins bind to poly-ubiquitinated proteins which, via binding to the phagophore-resident protein LC3, recruit their cargo to the growing autophagosome. Ubiquitination of mitochondrial surface proteins can occur via stabilisation of PINK1 (PTEN-induced kinase 1) on the mitochondrial surface, which recruits the E3 ubiquitin ligase, Parkin to mitochondrial membranes, thus promoting the ubiquitination and clearance of damaged mitochondria. The fully enclosed autophagosome is trafficked to and fuses with lysosomes. The contents of the subsequent autolysosome are degraded by lysosomal enzymes including hydrolases and proteases. mTORC1 integrates intra- and extra-cellular signals and drives cell growth as well as inhibiting autophagy. B) In young proliferating cells, mTORC1 carefully balances anabolic vs catabolic metabolism in response to the cellular environment. Both mTORC1 activity and autophagy are elevated in senescent cells while the specific process of mitophagy is reduced (see text for more details). Autophagy has a complex relationship with senescence and can both promote and inhibit senescence and senescence-associated phenotypes. For example in the context of OIS, autophagy induction can either promote or suppress acquisition of senescence while the specific role of mitophagy in OIS is currently less clear. Conversely, lifespan-extending interventions such as caloric restriction and rapamycin induce autophagy and mitophagy and relieve senescence-associated phenotypes.
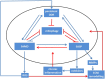
Fig. 3
The cell senescence signalling network. Signals downstream of the DDR cause mitochondrial dysfunction via p38MAPK and TGFβ, SASP and chronic inflammation via NF-κB and C/EBPβ and may directly or indirectly suppress autophagy and mitophagy. SASP and mitochondrial dysfunction feedback to maintain and enhance DNA damage at least partly via ROS generation. Many potential interactions within the network (indicated by red arrows) are still insufficiently understood. See text for further details.
Similar articles
- The role of mitochondria and mitophagy in cell senescence.
Ali T, Hussain F, Kayani HUR, Naeem M, Anjum F. Ali T, et al. Adv Protein Chem Struct Biol. 2023;136:93-115. doi: 10.1016/bs.apcsb.2023.03.001. Epub 2023 Mar 28. Adv Protein Chem Struct Biol. 2023. PMID: 37437987 - Targeting cellular senescence based on interorganelle communication, multilevel proteostasis, and metabolic control.
Cavinato M, Madreiter-Sokolowski CT, Büttner S, Schosserer M, Zwerschke W, Wedel S, Grillari J, Graier WF, Jansen-Dürr P. Cavinato M, et al. FEBS J. 2021 Jun;288(12):3834-3854. doi: 10.1111/febs.15631. Epub 2020 Dec 8. FEBS J. 2021. PMID: 33200494 Free PMC article. Review. - Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence.
Vizioli MG, Liu T, Miller KN, Robertson NA, Gilroy K, Lagnado AB, Perez-Garcia A, Kiourtis C, Dasgupta N, Lei X, Kruger PJ, Nixon C, Clark W, Jurk D, Bird TG, Passos JF, Berger SL, Dou Z, Adams PD. Vizioli MG, et al. Genes Dev. 2020 Mar 1;34(5-6):428-445. doi: 10.1101/gad.331272.119. Epub 2020 Jan 30. Genes Dev. 2020. PMID: 32001510 Free PMC article. - MAPK15 protects from oxidative stress-dependent cellular senescence by inducing the mitophagic process.
Franci L, Tubita A, Bertolino FM, Palma A, Cannino G, Settembre C, Rasola A, Rovida E, Chiariello M. Franci L, et al. Aging Cell. 2022 Jul;21(7):e13620. doi: 10.1111/acel.13620. Epub 2022 Jun 1. Aging Cell. 2022. PMID: 35642724 Free PMC article. - The critical role of mitophagy in cell senescence-mediated pulmonary fibrosis and potential therapeutic strategies.
Chen XY, Wang DM, Zhou Y, Liu L, Zhu T, Ran Z, Lu MH, Mu BR. Chen XY, et al. Mol Biol Rep. 2025 Jun 7;52(1):565. doi: 10.1007/s11033-025-10665-2. Mol Biol Rep. 2025. PMID: 40481901 Review.
Cited by
- PIN1 protects auditory hair cells from senescence via autophagy.
Lv Z, Zhang Y, Cao H, Liu Q, Feng X, Yin H, Wang B. Lv Z, et al. PeerJ. 2022 Nov 1;10:e14267. doi: 10.7717/peerj.14267. eCollection 2022. PeerJ. 2022. PMID: 36340199 Free PMC article. - Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement.
Tenchov R, Sasso JM, Wang X, Zhou QA. Tenchov R, et al. ACS Chem Neurosci. 2024 Jan 3;15(1):1-30. doi: 10.1021/acschemneuro.3c00531. Epub 2023 Dec 14. ACS Chem Neurosci. 2024. PMID: 38095562 Free PMC article. Review. - Effect of Pectin on the Expression of Proteins Associated with Mitochondrial Biogenesis and Cell Senescence in HT29-Human Colorectal Adenocarcinoma Cells.
Zamorano-León JJ, Ballesteros S, de Las Heras N, Alvarez-Sala L, de la Serna-Soto M, Zekri-Nechar K, Freixer G, Calvo-Rico B, Yang Z, García-García JM, Lahera V, López-Farré AJ. Zamorano-León JJ, et al. Prev Nutr Food Sci. 2019 Jun;24(2):187-196. doi: 10.3746/pnf.2019.24.2.187. Epub 2019 Jun 30. Prev Nutr Food Sci. 2019. PMID: 31328124 Free PMC article. - mTORC Inhibitors as Broad-Spectrum Therapeutics for Age-Related Diseases.
Walters HE, Cox LS. Walters HE, et al. Int J Mol Sci. 2018 Aug 8;19(8):2325. doi: 10.3390/ijms19082325. Int J Mol Sci. 2018. PMID: 30096787 Free PMC article. Review. - Reduced expression of mitochondrial complex I subunit Ndufs2 does not impact healthspan in mice.
McElroy GS, Chakrabarty RP, D'Alessandro KB, Hu YS, Vasan K, Tan J, Stoolman JS, Weinberg SE, Steinert EM, Reyfman PA, Singer BD, Ladiges WC, Gao L, Lopéz-Barneo J, Ridge K, Budinger GRS, Chandel NS. McElroy GS, et al. Sci Rep. 2022 Mar 25;12(1):5196. doi: 10.1038/s41598-022-09074-3. Sci Rep. 2022. PMID: 35338200 Free PMC article.
References
- Acosta J.C., O'loghlen A., Banito A., Guijarro M.V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N., Takatsu Y., Melamed J., D'adda Di Fagagna F., Bernard D., Hernando E., Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
- Akbar A.N., Henson S.M., Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37:866–876. - PubMed
Publication types
MeSH terms
Grants and funding
- 24009/CRUK_/Cancer Research UK/United Kingdom
- BB/F010966/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/I020748/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources