Synthesis, characterization, and antimicrobial properties of novel double layer nanocomposite electrospun fibers for wound dressing applications - PubMed (original) (raw)
Synthesis, characterization, and antimicrobial properties of novel double layer nanocomposite electrospun fibers for wound dressing applications
Alaa J Hassiba et al. Int J Nanomedicine. 2017.
Abstract
Herein, novel hybrid nanomaterials were developed for wound dressing applications with antimicrobial properties. Electrospinning was used to fabricate a double layer nanocomposite nanofibrous mat consisting of an upper layer of poly(vinyl alcohol) and chitosan loaded with silver nanoparticles (AgNPs) and a lower layer of polyethylene oxide (PEO) or polyvinylpyrrolidone (PVP) nanofibers loaded with chlorhexidine (as an antiseptic). The top layer containing AgNPs, whose purpose was to protect the wound site against environmental germ invasion, was prepared by reducing silver nitrate to its nanoparticulate form through interaction with chitosan. The lower layer, which would be in direct contact with the injured site, contained the antibiotic drug needed to avoid wound infections which would otherwise interfere with the healing process. Initially, the upper layer was electrospun, followed sequentially by electrospinning the second layer, creating a bilayer nanofibrous mat. The morphology of the nanofibrous mats was studied by scanning electron microscopy and transmission electron microscopy, showing successful nanofiber production. X-ray diffraction confirmed the reduction of silver nitrate to AgNPs. Fourier transform infrared spectroscopy showed a successful incorporation of the material used in the produced nanofibrous mats. Thermal studies carried out by thermogravimetric analysis indicated that the PVP-drug-loaded layer had the highest thermal stability in comparison to other fabricated nanofibrous mats. Antimicrobial activities of the as-synthesized nanofibrous mats against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans were determined using disk diffusion method. The results indicated that the PEO-drug-loaded mat had the highest antibacterial activity, warranting further attention for numerous wound-healing applications.
Keywords: activity; antimicrobial; biomedical; electrospinning; nanofibers; nanomaterials; wound dressing.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Figure 1
SEM image of fabricated electrospun mat. Notes: SEM image at 20,000× of electrospun nanofibers of PVA/chitosan/AgNPs blends of 12 wt%/0.6 wt%/0.9 wt%, respectively, using the following electrospinning conditions of 10 cm, 18 kV, and 0.3 mL/h. The EDS was collected on the PVA/chitosan/AgNPs. Abbreviations: SEM, scanning electron microscopy; PVA, poly(vinyl alcohol); AgNPs, silver nanoparticles; EDS, energy dispersive spectrum; wt, weight; HV, high voltage; Mag, magnification; WD, working distance.
Figure 2
SEM image of fabricated electrospun mat. Note: SEM image at 20,000× of electrospun nanofibers of PVP 12% (wt/v) using the following electrospinning conditions of 10 cm, 18 kV, and 0.3 mL/h. Abbreviations: SEM, scanning electron microscopy; PVP, polyvinylpyrrolidone; v, volume; wt, weight; HV, high voltage; Mag, magnification; WD, working distance.
Figure 3
SEM image of fabricated electrospun mat. Note: SEM image at 1,000× of electrospun nanofibers of PEO 8% (wt/v) using the following electrospinning conditions of 10 cm, 18 kV, and 0.3 mL/h. Abbreviations: SEM, scanning electron microscopy; PEO, polyethylene oxide; v, volume; wt, weight; HV, high voltage; Mag, magnification; WD, working distance.
Figure 4
TEM image showing AgNPs on a nanofiber. Abbreviations: TEM, transmission electron microscopy; AgNPs, silver nanoparticles.
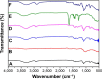
Figure 5
Comparative result of FT-IR spectra for all fabricated samples. Notes: FT-IR shown is for (A) PVA, (B) chitosan, (C) PVA/chitosan, (D) PVA/chitosan/AgNPs, (E) PVA/chitosan/AgNPs/PVP/chlorhexidine, and (F) PVA/chitosan/AgNPs/PEO/chlorhexidine. Abbreviations: FT-IR, Fourier transform infrared spectroscopy; PVA, poly(vinyl alcohol); AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; PEO, polyethylene oxide.
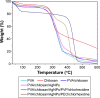
Figure 6
Comparative result of TGA spectra for all samples. Notes: TGA shown is for PVA, chitosan, PVA/chitosan, PVA/chitosan/AgNPs, PVA/chitosan/AgNPs/PVP/chlorhexidine, and PVA/chitosan/AgNPs/PEO/chlorhexidine. Abbreviations: TGA, thermogravimetric analysis; PVA, poly(vinyl alcohol); AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; PEO, polyethylene oxide.
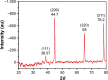
Figure 7
XRD spectra for electrospun PVA/chitosan/AgNPs. Note: The four diffraction peaks are ascribed to corresponding reflection planes of FCC structure of Ag phase. Abbreviations: XRD, X-ray diffraction; PVA, poly(vinyl alcohol); AgNPs, silver nanoparticles; FCC, face-centered cubic; au, atomic unit; Ag, silver.
Figure 8
Antimicrobial susceptibility disk diffusion test. Notes: (1) PVA/chitosan (control), (2) PVA/chitosan/AgNPs, (3) PVP-drug loaded, and (4) PEO–drug loaded against (A) Staphylococcus aureus, (B) Escherichia coli, (C) Pseudomonas aeruginosa, and (D) Candid albicans. Abbreviations: PVA, poly(vinyl alcohol); AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; PEO, polyethylene oxide.
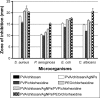
Figure 9
Diameter of zones of inhibition for PVA/chitosan, PVA/chitosan/AgNPs, PVP/chlorhexidine, and PEO/chlorhexidine against S. aureus, P. aeruginosa, E. coli, and C. albicans. Abbreviations: PVA, poly(vinyl alcohol); AgNPs, silver nanoparticles; PVP, polyvinylpyrrolidone; PEO, polyethylene oxide; S. aureus, Staphylococcus aureus; P. aeruginosa, Pseudomonas aeruginosa; E. coli, Escherichia coli; C. albicans, Candida albicans.
Similar articles
- Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing.
Ahmed R, Tariq M, Ali I, Asghar R, Noorunnisa Khanam P, Augustine R, Hasan A. Ahmed R, et al. Int J Biol Macromol. 2018 Dec;120(Pt A):385-393. doi: 10.1016/j.ijbiomac.2018.08.057. Epub 2018 Aug 12. Int J Biol Macromol. 2018. PMID: 30110603 - A novel bioactive quaternized chitosan and its silver-containing nanocomposites as a potent antimicrobial wound dressing: Structural and biological properties.
Rahimi M, Ahmadi R, Samadi Kafil H, Shafiei-Irannejad V. Rahimi M, et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:360-369. doi: 10.1016/j.msec.2019.03.092. Epub 2019 Mar 26. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31029329 - Electrospun PCL/mupirocin and chitosan/lidocaine hydrochloride multifunctional double layer nanofibrous scaffolds for wound dressing applications.
Li X, Wang C, Yang S, Liu P, Zhang B. Li X, et al. Int J Nanomedicine. 2018 Sep 10;13:5287-5299. doi: 10.2147/IJN.S177256. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30237715 Free PMC article. - An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings.
Pilehvar-Soltanahmadi Y, Dadashpour M, Mohajeri A, Fattahi A, Sheervalilou R, Zarghami N. Pilehvar-Soltanahmadi Y, et al. Mini Rev Med Chem. 2018 Feb 14;18(5):414-427. doi: 10.2174/1389557517666170308112147. Mini Rev Med Chem. 2018. PMID: 28271816 Review. - An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process.
Miguel SP, Sequeira RS, Moreira AF, Cabral CSD, Mendonça AG, Ferreira P, Correia IJ. Miguel SP, et al. Eur J Pharm Biopharm. 2019 Jun;139:1-22. doi: 10.1016/j.ejpb.2019.03.010. Epub 2019 Mar 7. Eur J Pharm Biopharm. 2019. PMID: 30853442 Review.
Cited by
- Fabrication and characterization of polyvinyl alcohol-chitosan composite nanofibers for carboxylesterase immobilization to enhance the stability of the enzyme.
Kaur H, Singh S, Rode S, Chaudhary PK, Khan NA, Ramamurthy PC, Gupta DN, Kumar R, Das J, Sharma AK. Kaur H, et al. Sci Rep. 2024 Aug 23;14(1):19615. doi: 10.1038/s41598-024-67913-x. Sci Rep. 2024. PMID: 39179653 Free PMC article. - Antifungal Activity of Chitosan/Poly(Ethylene Oxide) Blend Electrospun Polymeric Fiber Mat Doped with Metallic Silver Nanoparticles.
Murillo L, Rivero PJ, Sandúa X, Pérez G, Palacio JF, Rodríguez RJ. Murillo L, et al. Polymers (Basel). 2023 Sep 8;15(18):3700. doi: 10.3390/polym15183700. Polymers (Basel). 2023. PMID: 37765554 Free PMC article. - Current status and progress in research on dressing management for diabetic foot ulcer.
Jiang P, Li Q, Luo Y, Luo F, Che Q, Lu Z, Yang S, Yang Y, Chen X, Cai Y. Jiang P, et al. Front Endocrinol (Lausanne). 2023 Aug 17;14:1221705. doi: 10.3389/fendo.2023.1221705. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37664860 Free PMC article. Review. - Multi-material electrospinning: from methods to biomedical applications.
Xing J, Zhang M, Liu X, Wang C, Xu N, Xing D. Xing J, et al. Mater Today Bio. 2023 Jun 23;21:100710. doi: 10.1016/j.mtbio.2023.100710. eCollection 2023 Aug. Mater Today Bio. 2023. PMID: 37545561 Free PMC article. Review. - Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management.
Sabarees G, Velmurugan V, Tamilarasi GP, Alagarsamy V, Raja Solomon V. Sabarees G, et al. Polymers (Basel). 2022 Sep 23;14(19):3994. doi: 10.3390/polym14193994. Polymers (Basel). 2022. PMID: 36235942 Free PMC article. Review.
References
- Abrigo M, McArthur SL, Kingshott P. Electrospun nanofibers as dressings for chronic wound care: advances, challenges, and future prospects. Macromol Biosci. 2014;14(6):772–792. - PubMed
- Li M, Mondrinos MJ, Gandhi MR, Ko FK, Weiss AS, Lelkes PI. Electrospun protein fibers as matrices for tissue engineering. Biomaterials. 2005;26(30):5999–6008. - PubMed
- Pham QP, Sharma U, Mikos AG. Electrospun poly (epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. - PubMed
- Shao S, Li L, Yang G, et al. Controlled green tea polyphenols release from electrospun PCL/MWCNTs composite nanofibers. Int J Pharm. 2011;421(2):310–320. - PubMed
- Ignatova M, Rashkov I, Manolova N. Drug-loaded electrospun materials in wound-dressing applications and in local cancer treatment. Expert Opin Drug Deliv. 2013;10(4):469–483. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources