Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model - PubMed (original) (raw)
Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model
Rebecca M Nisbet et al. Brain. 2017.
Abstract
Alzheimer's disease is characterized by the deposition of amyloid-β as extracellular plaques and hyperphosphorylated tau as intracellular neurofibrillary tangles. Tau pathology characterizes not only Alzheimer's disease, but also many other tauopathies, presenting tau as an attractive therapeutic target. Passive tau immunotherapy has been previously explored; however, because only a small fraction of peripherally delivered antibodies crosses the blood-brain barrier, enters the brain and engages with tau that forms intracellular aggregates, more efficient ways of antibody delivery and neuronal uptake are warranted. In the brain, tau exists as multiple isoforms. Here, we investigated the efficacy of a novel 2N tau isoform-specific single chain antibody fragment, RN2N, delivered by passive immunization in the P301L human tau transgenic pR5 mouse model. We demonstrate that, in treated mice, RN2N reduces anxiety-like behaviour and phosphorylation of tau at distinct sites. When administration of RN2N was combined with focused ultrasound in a scanning mode (scanning ultrasound), RN2N delivery into the brain and uptake by neurons were markedly increased, and efficacy was significantly enhanced. Our study provides evidence that scanning ultrasound is a viable tool to enhance the delivery of biologics across the blood-brain barrier and improve therapeutic outcomes and further presents single-chain antibodies as an alternative to full-length antibodies.
Keywords: Alzheimer’s disease; blood–brain barrier; dementia; neurofibrillary tangles; tau.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
Figure 1
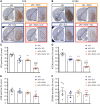
RN2N treatment in combination with SUS reduces anxiety-like behaviour in pR5 tau transgenic mice. (A) Schematic of ultrasound treatment using a transducer in scanning (SUS) mode in order to achieve microbubble-assisted opening of the blood–brain barrier. (B) Female pR5 mice at 4.5 months of age were randomly assigned to one of four groups: pR5, pR5 + SUS, pR5 + RN2N and pR5 + RN2N + SUS and treated as indicated (X) once a week for 4 weeks. A group of wild-type (WT) littermate controls did not undergo any treatment. Upon treatment completion, mice were analysed on the elevated plus maze (EPM), and then sacrificed. (C) The elevated plus maze is an elevated cross-shaped apparatus with a central square and 15 cm long × 5 cm wide closed arms with a 15 cm wall and open arms with unprotected edges. (D) The mean positional heat map within the elevated plus maze for each treatment group. (E) pR5 mice (n = 6) spend significantly less time in the open arms compared to wild-type mice (n = 7) (****P < 0.0001). No difference was observed in the pR5 + SUS group (n = 6) compared to the pR5 mice. However, mice in the pR5 + RN2N group (n = 6) and those in the pR5 + RN2N + SUS group (n = 5) spent significantly more time in the open arms than pR5 mice (*P = 0.03 and ***P = 0.0002, respectively), indicating a reduction in anxiety-like behaviour (mean ± SEM; one-way ANOVA with Dunnett’s multiple comparison test).
Figure 2
Delivery of RN2N in combination with SUS significantly reduces phosphorylated tau levels in the amygdala. (A) Representative images of the AT8 phosphorylated tau immunoreactivity observed in a full brain section and amygdala of pR5 mice in each treatment group (Scale bar = 100 μm). (B) Representative images of the AT180 phosphorylated tau immunoreactivity observed in the amygdala of pR5 mice in each treatment group (Scale bar = 100 μm). (C) AT8 phosphorylated tau in the amygdala was significantly reduced in all treatment groups compared to the pR5 group (****P ≤ 0.0001). Comparison of the pR5 + RN2N + SUS group to the pR5 + SUS and pR5 + RN2N groups demonstrates a further reduction in AT8 tau-positive area in the amygdala (####P ≤ 0.0001). (D) AT180 phosphorylated tau in the amygdala was significantly reduced in all treatment groups compared to the pR5 group (****P ≤ 0.0001). Comparison of the pR5 + RN2N + SUS group to the pR5 + SUS and pR5 + RN2N groups demonstrates a further reduction in AT180 tau-positive area in the amygdala (###P = 0.0002). (E) 12E8 phosphorylated tau in the amygdala was significantly reduced in the RN2N + SUS group compared to the pR5 group (**P < 0.01), and was reduced in comparison to pR5 + SUS and pR5 + RN2N groups (#P ≤ 0.05). (F) Ser404 phosphorylated tau in the amygdala was not significantly reduced in any treatment group compared to the pR5 group (mean ± SEM, one-way ANOVA with Tukey’s multiple comparison test, n = 5).
Figure 3
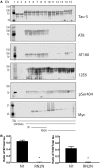
RN2N inhibits GSK3β phosphorylation of tau in vitro . (A) GSK3β phosphorylates tau at multiple serine and threonine epitopes resulting in a molecular weight shift of tau. Lane 1: molecular weight marker; lanes 2 and 3: tau alone; lanes 4 and 5: tau incubated with GSK3β; lanes 6 and 7: tau incubated with GSK3β in the presence of 10 μM N1 control scFv; lanes 8 and 9: tau incubated with GSK3β in the presence of 1 μM N1 control scFv; lanes 10 and 11: tau incubated with GSK3β in the presence of 10 μM RN2N; lanes 12 and 13: tau incubated with GSK3β in the presence of 1 μM RN2N; and lanes 14 and 15: tau incubated with GSK3β in the presence of 0.1 μM RN2N. Co-treatment of tau and GSK3β with RN2N leads to reduced phosphorylation at the AT8 and AT180 epitopes, quantified in B, with no change to 12E8 or pSer404 epitopes. Blotting for myc indicates the presence of scFv. (B) Quantification of the reduction of phosphorylation of the AT8 and AT180 epitopes with 10 μM RN2N compared to 10 μM N1 control scFv (P < 0.05) (mean ± SEM, _t_-test).
Figure 4
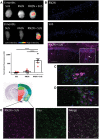
SUS enhances RN2N scFv delivery across the blood–brain barrier and into neurons. pR5 mice under anaesthesia were injected retro-orbitally with either microbubbles only (SUS), RN2N conjugated to Alexa 647 only (RN2N) or a combination of both (RN2N + SUS). SUS was then applied to SUS and RN2N + SUS groups. After 30 min mice were sacrificed and tissue analysed by fluorescence imaging. (A) Fluorescence imaging indicates widespread brain uptake of RN2N in 6-month and 8-month-old RN2N + SUS treated mice. Quantification of the fluorescent intensity demonstrates an approximate 11-fold increase in scFv uptake in the RN2N + SUS treated mice (mean = 662 ± 119) compared to the RN2N only treated mice (mean = 58 ± 32) (grouping 6-month-old mice = red squares; and 8-month-old mice = black circles; mean ± SEM, P < 0.0001, one-way ANOVA with Tukey’s post-test). (B) Post-sectioning, RN2N was only detectable in the RN2N + SUS treated mice as shown for the CA1 region of the hippocampus. Inset: Zoomed-in image of RN2N + SUS treated mouse brain tissue with RN2N observed within neuronal cell bodies and apical dendrites (arrow). (C) Co-immunofluorescence staining for EEA1 (green) and RN2N (magenta) and (D) LAMP1 (green) and RN2N (magenta) indicates that the internalized RN2N is not confined to endosomes or lysosomes (Scale bar = 10 μm). (E) In the RN2N + SUS treated mice, RN2N is also observed in the amygdala where it co-localized with HT7 staining (human tau). Coronal image sourced from Allen Mouse Brain Atlas (2004) (Scale bar = 50 μm).
Similar articles
- Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice.
Ittner A, Bertz J, Suh LS, Stevens CH, Götz J, Ittner LM. Ittner A, et al. J Neurochem. 2015 Jan;132(1):135-45. doi: 10.1111/jnc.12821. Epub 2014 Aug 1. J Neurochem. 2015. PMID: 25041093 - Tau antibody isotype induces differential effects following passive immunisation of tau transgenic mice.
Bajracharya R, Brici D, Bodea LG, Janowicz PW, Götz J, Nisbet RM. Bajracharya R, et al. Acta Neuropathol Commun. 2021 Mar 12;9(1):42. doi: 10.1186/s40478-021-01147-0. Acta Neuropathol Commun. 2021. PMID: 33712083 Free PMC article. - Anti-tau conformational scFv MC1 antibody efficiently reduces pathological tau species in adult JNPL3 mice.
Vitale F, Giliberto L, Ruiz S, Steslow K, Marambaud P, d'Abramo C. Vitale F, et al. Acta Neuropathol Commun. 2018 Aug 22;6(1):82. doi: 10.1186/s40478-018-0585-2. Acta Neuropathol Commun. 2018. PMID: 30134961 Free PMC article. - Immunotherapy for tauopathies.
Gu J, Sigurdsson EM. Gu J, et al. J Mol Neurosci. 2011 Nov;45(3):690-5. doi: 10.1007/s12031-011-9576-5. Epub 2011 Jul 8. J Mol Neurosci. 2011. PMID: 21739165 Free PMC article. Review. - A walk through tau therapeutic strategies.
Jadhav S, Avila J, Schöll M, Kovacs GG, Kövari E, Skrabana R, Evans LD, Kontsekova E, Malawska B, de Silva R, Buee L, Zilka N. Jadhav S, et al. Acta Neuropathol Commun. 2019 Feb 15;7(1):22. doi: 10.1186/s40478-019-0664-z. Acta Neuropathol Commun. 2019. PMID: 30767766 Free PMC article. Review.
Cited by
- Applications of focused ultrasound-mediated blood-brain barrier opening.
Gorick CM, Breza VR, Nowak KM, Cheng VWT, Fisher DG, Debski AC, Hoch MR, Demir ZEF, Tran NM, Schwartz MR, Sheybani ND, Price RJ. Gorick CM, et al. Adv Drug Deliv Rev. 2022 Dec;191:114583. doi: 10.1016/j.addr.2022.114583. Epub 2022 Oct 19. Adv Drug Deliv Rev. 2022. PMID: 36272635 Free PMC article. Review. - Applications of focused ultrasound in the brain: from thermoablation to drug delivery.
Meng Y, Hynynen K, Lipsman N. Meng Y, et al. Nat Rev Neurol. 2021 Jan;17(1):7-22. doi: 10.1038/s41582-020-00418-z. Epub 2020 Oct 26. Nat Rev Neurol. 2021. PMID: 33106619 Review. - A multicellular brain spheroid model for studying the mechanisms and bioeffects of ultrasound-enhanced drug penetration beyond the blood‒brain barrier.
Paranjape AN, D'Aiuto L, Zheng W, Chen X, Villanueva FS. Paranjape AN, et al. Sci Rep. 2024 Jan 22;14(1):1909. doi: 10.1038/s41598-023-50203-3. Sci Rep. 2024. PMID: 38253669 Free PMC article. - Multimodal analysis of aged wild-type mice exposed to repeated scanning ultrasound treatments demonstrates long-term safety.
Blackmore DG, Turpin F, Mohamed AZ, Zong F, Pandit R, Pelekanos M, Nasrallah F, Sah P, Bartlett PF, Götz J. Blackmore DG, et al. Theranostics. 2018 Nov 29;8(22):6233-6247. doi: 10.7150/thno.27941. eCollection 2018. Theranostics. 2018. PMID: 30613294 Free PMC article. - Evaluating the safety profile of focused ultrasound and microbubble-mediated treatments to increase blood-brain barrier permeability.
McMahon D, Poon C, Hynynen K. McMahon D, et al. Expert Opin Drug Deliv. 2019 Feb;16(2):129-142. doi: 10.1080/17425247.2019.1567490. Epub 2019 Jan 29. Expert Opin Drug Deliv. 2019. PMID: 30628455 Free PMC article. Review.
References
- Afadzi M, Strand SP, Nilssen EA, Masoy SE, Johansen TF, Hansen R et al. . Mechanisms of the ultrasound-mediated intracellular delivery of liposomes and dextrans. IEEE Trans Ultrason Ferroelectr Freq Control 2013; 60: 21–33. - PubMed
- Alonso A, Reinz E, Fatar M, Jenne J, Hennerici MG, Meairs S. Neurons but not glial cells overexpress ubiquitin in the rat brain following focused ultrasound-induced opening of the blood-brain barrier. Neuroscience 2010; 169: 116–24. - PubMed
- Amniai L, Lippens G, Landrieu I. Characterization of the AT180 epitope of phosphorylated Tau protein by a combined nuclear magnetic resonance and fluorescence spectroscopy approach. Biochem Biophys Res Commun 2011; 412: 743–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical