The Drug Repurposing Hub: a next-generation drug library and information resource - PubMed (original) (raw)
. 2017 Apr 7;23(4):405-408.
doi: 10.1038/nm.4306.
Steven M Corsello 1 2 3, Zihan Liu 1, Joshua Gould 1, Patrick McCarren 1, Jodi E Hirschman 1, Stephen E Johnston 1, Anita Vrcic 1, Bang Wong 1, Mariya Khan 1, Jacob Asiedu 1, Rajiv Narayan 1, Christopher C Mader 1, Aravind Subramanian 1, Todd R Golub 1 3 4 5
Affiliations
- PMID: 28388612
- PMCID: PMC5568558
- DOI: 10.1038/nm.4306
The Drug Repurposing Hub: a next-generation drug library and information resource
Steven M Corsello et al. Nat Med. 2017.
No abstract available
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures
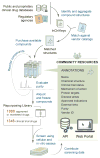
Figure 1. The Repurposing Hub workflow for drug library creation
Drugs were identified, purchased, confirmed, and fully annotated. The final QC-confirmed library contains 1,988 drugs that are approved or marketed for clinical use around the world and 1,348 drugs that reached Phase 1–3 in clinical development.
Figure 2. Repurposing Library contents
A) Highest clinical phase achieved by each compound. 3,422 drugs in the Library have reached clinical use as of November 2016. B) Number of compounds per target category. Compounds with multiple targets may be indicated in more than one category. C) Number of approved drugs for indications within each listed disease area. D) Classification of proteins targeted by Library drugs. Protein function hierarchy is shown with increasing specificity of function corresponding to distance from the figure center. The relative area of each segment is proportional to the fraction of the Repurposing Library targeting each protein class. As expected, the Library is enriched in drugs targeting kinases, GPCRs, and ion channels.
Similar articles
- DrugR+: A comprehensive relational database for drug repurposing, combination therapy, and replacement therapy.
Masoudi-Sobhanzadeh Y, Omidi Y, Amanlou M, Masoudi-Nejad A. Masoudi-Sobhanzadeh Y, et al. Comput Biol Med. 2019 Jun;109:254-262. doi: 10.1016/j.compbiomed.2019.05.006. Epub 2019 May 8. Comput Biol Med. 2019. PMID: 31096089 - Drug databases and their contributions to drug repurposing.
Masoudi-Sobhanzadeh Y, Omidi Y, Amanlou M, Masoudi-Nejad A. Masoudi-Sobhanzadeh Y, et al. Genomics. 2020 Mar;112(2):1087-1095. doi: 10.1016/j.ygeno.2019.06.021. Epub 2019 Jun 18. Genomics. 2020. PMID: 31226485 Review. - DrugMap Central: an on-line query and visualization tool to facilitate drug repositioning studies.
Fu C, Jin G, Gao J, Zhu R, Ballesteros-Villagrana E, Wong ST. Fu C, et al. Bioinformatics. 2013 Jul 15;29(14):1834-6. doi: 10.1093/bioinformatics/btt279. Epub 2013 May 15. Bioinformatics. 2013. PMID: 23681121 Free PMC article. - Expanding the scope of drug repurposing in pediatrics: the Children's Pharmacy Collaborative.
Blatt J, Farag S, Corey SJ, Sarrimanolis Z, Muratov E, Fourches D, Tropsha A, Janzen WP. Blatt J, et al. Drug Discov Today. 2014 Nov;19(11):1696-1698. doi: 10.1016/j.drudis.2014.08.003. Epub 2014 Aug 19. Drug Discov Today. 2014. PMID: 25149597 Free PMC article. - Web-based drug repurposing tools: a survey.
Sam E, Athri P. Sam E, et al. Brief Bioinform. 2019 Jan 18;20(1):299-316. doi: 10.1093/bib/bbx125. Brief Bioinform. 2019. PMID: 29028878 Review.
Cited by
- Worldwide analysis of actionable genomic alterations in lung cancer and targeted pharmacogenomic strategies.
Echeverría-Garcés G, Ramos-Medina MJ, González A, Vargas R, Cabrera-Andrade A, Armendáriz-Castillo I, García-Cárdenas JM, Ramírez-Sánchez D, Altamirano-Colina A, Echeverría-Espinoza P, Freire MP, Ocaña-Paredes B, Rivera-Orellana S, Guerrero S, Quiñones LA, López-Cortés A. Echeverría-Garcés G, et al. Heliyon. 2024 Sep 5;10(17):e37488. doi: 10.1016/j.heliyon.2024.e37488. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296198 Free PMC article. - An In Vitro Investigation of the Antiproliferative and Antimetastatic Effects of Levosimendan: Potential Drug Repurposing for Cervical Cancer.
Schelz Z, Muddather HF, Jaski FS, Bózsity N, Zupkó I. Schelz Z, et al. Curr Issues Mol Biol. 2024 Jun 27;46(7):6566-6579. doi: 10.3390/cimb46070391. Curr Issues Mol Biol. 2024. PMID: 39057033 Free PMC article. - IL10RB as a key regulator of COVID-19 host susceptibility and severity.
Voloudakis G, Hoffman G, Venkatesh S, Lee KM, Dobrindt K, Vicari JM, Zhang W, Beckmann ND, Jiang S, Hoagland D, Bian J, Gao L, Corvelo A, Cho K, Lee JS, Iyengar SK, Luoh SW, Akbarian S, Striker R, Assimes TL, Schadt EE, Merad M, tenOever BR, Charney AW; Mount Sinai COVID-19 Biobank; VA Million Veteran Program COVID-19 Science Initiative; Brennand KJ, Lynch JA, Fullard JF, Roussos P. Voloudakis G, et al. medRxiv [Preprint]. 2021 Jun 2:2021.05.31.21254851. doi: 10.1101/2021.05.31.21254851. medRxiv. 2021. PMID: 34100031 Free PMC article. Updated. Preprint. - Strategies to Enhance Logic Modeling-Based Cell Line-Specific Drug Synergy Prediction.
Niederdorfer B, Touré V, Vazquez M, Thommesen L, Kuiper M, Lægreid A, Flobak Å. Niederdorfer B, et al. Front Physiol. 2020 Jul 28;11:862. doi: 10.3389/fphys.2020.00862. eCollection 2020. Front Physiol. 2020. PMID: 32848834 Free PMC article. - A High Content Screen for Mucin-1-Reducing Compounds Identifies Fostamatinib as a Candidate for Rapid Repurposing for Acute Lung Injury during the COVID-19 pandemic.
Alimova M, Sidhom EH, Satyam A, Dvela-Levitt M, Melanson M, Chamberlain BT, Alper SL, Santos J, Gutierrez J, Subramanian A, Grinkevich E, Bricio ER, Kim C, Clark A, Watts A, Thompson R, Marshall J, Pablo JL, Coraor J, Roignot J, Vernon KA, Keller K, Campbell A, Emani M, Racette M, Bazua-Valenti S, Padovano V, Weins A, McAdoo SP, Tam FWK, Ronco L, Wagner F, Tsokos GC, Shaw JL, Greka A. Alimova M, et al. bioRxiv [Preprint]. 2020 Jun 30:2020.06.30.180380. doi: 10.1101/2020.06.30.180380. bioRxiv. 2020. PMID: 32637960 Free PMC article. Updated. Preprint.
References
- Nosengo N. Can you teach old drugs new tricks? Nature. 2016;534:314–316. - PubMed
- Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J. Clinical development success rates for investigational drugs. Nat Biotechnol. 2014;32:40–51. - PubMed
- Goldstein I, et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338:1397–1404. - PubMed
- Palumbo A, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111:3968–3977. - PubMed
- Lamb J, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- KL2 TR001100/TR/NCATS NIH HHS/United States
- U54 HG006093/HG/NHGRI NIH HHS/United States
- T32 CA009172/CA/NCI NIH HHS/United States
- U01 HG008699/HG/NHGRI NIH HHS/United States
- U54 HL127366/HL/NHLBI NIH HHS/United States
- HHMI_/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources