Determining the bacterial cell biology of Planctomycetes - PubMed (original) (raw)
Review
doi: 10.1038/ncomms14853.
Margarete Schüler 2, Greta Reintjes 3, Olga Jeske 1, Muriel C F van Teeseling 4 5, Mareike Jogler 1, Patrick Rast 1, Daniela Borchert 1, Damien P Devos 6, Martin Kucklick 7 8, Miroslava Schaffer 2, Roberto Kolter 9, Laura van Niftrik 4, Susanne Engelmann 7 8, Rudolf Amann 3, Manfred Rohde 7, Harald Engelhardt 2, Christian Jogler 1 4
Affiliations
- PMID: 28393831
- PMCID: PMC5394234
- DOI: 10.1038/ncomms14853
Review
Determining the bacterial cell biology of Planctomycetes
Christian Boedeker et al. Nat Commun. 2017.
Abstract
Bacteria of the phylum Planctomycetes have been previously reported to possess several features that are typical of eukaryotes, such as cytosolic compartmentalization and endocytosis-like macromolecule uptake. However, recent evidence points towards a Gram-negative cell plan for Planctomycetes, although in-depth experimental analysis has been hampered by insufficient genetic tools. Here we develop methods for expression of fluorescent proteins and for gene deletion in a model planctomycete, Planctopirus limnophila, to analyse its cell organization in detail. Super-resolution light microscopy of mutants, cryo-electron tomography, bioinformatic predictions and proteomic analyses support an altered Gram-negative cell plan for Planctomycetes, including a defined outer membrane, a periplasmic space that can be greatly enlarged and convoluted, and an energized cytoplasmic membrane. These conclusions are further supported by experiments performed with two other Planctomycetes, Gemmata obscuriglobus and Rhodopirellula baltica. We also provide experimental evidence that is inconsistent with endocytosis-like macromolecule uptake; instead, extracellular macromolecules can be taken up and accumulate in the periplasmic space through unclear mechanisms.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
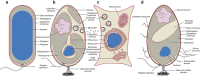
Figure 1. Cellular structures of a typical bacterium, planctomycetes and eukaryotes.
(a) A typical Gram-negative prokaryote is surrounded by an outer membrane (OM), a peptidoglycan (PG) cell wall and the cytoplasmic membrane (CM). The DNA forms the nucleoid and occupies a major portion of the cytoplasm. (b) Planctomycetes have been previously proposed to possess a unique cell plan. It was thought that PG was absent and replaced by a proteinaceous cell wall instead. The outermost membrane (OM) has been interpreted as CM, while an additional intracytoplasmic membrane (ICM) would divide the cytoplasm into a paryphoplasm and a pirellulosome. While the nucleoid of all Planctomycetes is highly condensed, G. obscuriglobus was proposed to contain an additional double membrane surrounding the DNA, similarly to the eukaryotic nucleus. Other planctomycetal species, the anammox bacteria, have additional subcellular structures such as the anammoxosome, an organelle responsible for the generation of energy. Most strikingly, Planctomycetes were reported to perform endocytosis-like uptake of macromolecules employing membrane-coat-like proteins that structurally resemble eukaryotic membrane-coat proteins such as clathrin. (c) A typical eukaryotic cell with membranous organelles and the ability to perform endocytosis. (d) Recent work and this study substantiate the view that Planctomycetes possess a Gram-negative cell architecture. The cells show a remarkable tendency for massive invaginations of the cytoplasmic membrane. Crateriform structures are found at sites of contact between the inner and outer membrane. Uptake of large molecules does not appear to be mediated by vesicles in P. limnophila and G. obscuriglobus.
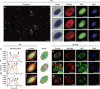
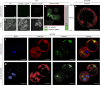
Figure 2. Planctopirus limnophila cells possess diverse enlargements of the periplasmic space.
(a–m) P. limnophila cells were engineered to produce GFP as cytoplasmic marker (green), while nucleoids and membranes were stained blue (DAPI) and red (wide field (WF): FM4–64, super resolution structured illumination microscopy (SR-SIM): Nile Red), respectively. 1,838 cells in 10 fields of view were analysed employing wide field microscopy (a: overview of one field of view; see Supplementary Fig. 1a,b for details). Two distinct phenotypes were observed: 27.6% of the cells comprised a typical Gram-negative membrane and cytoplasm staining patterns (b); 72.4% of the analysed cells showed additional red foci (c,d). Line profiles of fluorescence intensity were plotted longitudinal (e–j: along white arrows) and either two (e: black arrowheads) or multiple (f,g: red arrowheads) membrane staining maxima (e,f: red lines) were observed. The GFP signal maxima (e–g: green lines) never overlapped with the red outer membrane maxima (black arrowheads) while it did with additional membrane maxima (f,g: green and red arrowheads). If representative cells are considered (b–d), the corresponding overlays in (i,j) show colocalization (yellow) of the cytoplasm (green) and the membrane (red) signals in such cells. SR-SIM microscopy resolves these foci as invaginations of the innermost membrane into the cytoplasm (k–m: overlay and Nile Red) as schematically illustrated in the model. Scale bars, 5 μm (a) and 1 μm (b–m).
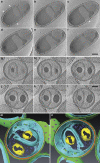
Figure 3. Cryo-electron tomography of Planctopirus limnophila cells.
(a–f) Representative slices of a whole cell tomogram with inter-slice distances of 71, 71, 64, 71 and 64 nm from top left to bottom right (Supplementary Movie 1). The membrane is heavily invaginated and creates a huge periplasmic space. Crateriform structures (‘pits') in the cell wall are always in close contact to the cell membrane (arrowheads) and are missing at sites of invagination (asterisk). (g–l) Slices of a tomogram of a cell thinned to 320 nm by focused ion beam (FIB) micromachining. Inter-slice distances are 41 nm throughout. Example of a cell with complex membrane invaginations creating a ‘channel system' of the periplasm inside the inner cell volume. Scale bars, 250 nm. (m,n) Surface-rendered reconstruction of the cell in (g–l) visualized in two different angles (m,n). Colour code: cell membrane (light blue), PG (orange), outer membrane (green), crateriform structures (purple), granules (yellow), ribosomes (dark blue).
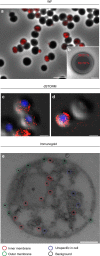
Figure 4. The innermost planctomycetal membrane is energized.
(a) Overlay of the wide field (WF) fluorescence signal from a representative ATPase localization experiment (red, anti-Na+-F1F0-ATPase antibody) with the corresponding phase contrast (phaco) micrograph of G. obscuriglobus cells. Scale bar, 5 μm. (b) Among 50 analysed cells in five fields of view, 39.21% of the fluorescence signals were detected in the outer rim, as indicated by the schematic drawing overlaying a phaco micrograph of a single G. obscuriglobus cell. 60.79% of the signals were detected within the cytoplasmic area of cells (_t_-test: _P_= 0.0001, see Supplementary Fig. 5a,b for details). Scale bar, 1 μm. (c,d) Representative overlays of super-resolution direct stochastic optical reconstruction microscopic (dSTORM) ATPase localization experiments (red, anti-Na+-F1F0-ATPase antibody) with the corresponding differential interference contrast micrograph of G. obscuriglobus cells. Scale bars, 1 μm. (e) Immunogold-labelling of the ATPase (black dots, anti-Na+-F1F0-ATPase antibody) in high pressure-frozen and freeze-substituted G. obscuriglobus cells (see Supplementary Fig. 5c,d for detail). The micrograph shows four different localizations of gold particles which are either associated with the inner membrane (red circles), the outer membrane (green circles) or show non-specific localizations either within the cell (blue circles) or outside of the cell (black circles). Scale bar, 0.2 μm.
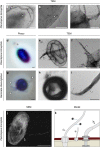
Figure 5. Gemmata obscuriglobus takes up high-molecular weight dextran polysaccharides.
(a–c) Wide field (WF) microscopy of G. obscuriglobus cells that were either untreated (Ctr) or fed with fluorescein-labelled 40 kDa dextran (Dextran). Prior to addition of dextran cell samples were either poisoned with carbonyl cyanide m-chlorophenyl hydrazon (+CCCP) or left untreated (−CCCP). Epifluorescence overview micrographs (a) were combined with differential interference contrast images of the same field of view (b). Dextran fluorescence signals (green) were exclusively detected in −CCCP cells, indicating an active uptake. Scale bars, 10 μm. (c) Magnification of the red framed section in a shows distinct foci of dextran accumulation. Scale bar, 10 μm. (d) While representative images are shown in (a–c), in total 1,065 cells among 7 fields of view were analysed and 23% of them showed dextran uptake (error bars indicate s.d.). In contrast, among 816 CCCP treated cells not a single uptake event was observed. (e) Super-resolution direct stochastic optical reconstruction microscopic (dSTORM) analysis of G. obscuriglobus cells fed with Alexa 467-labelled 10 kDa dextran. The red fluorescence signals show an almost homogeneous distribution of dextran in some areas of the cell (see Supplementary Fig. 8a–f for details). Scale bar, 1 μm. (f) Super-resolution structured illumination microscopy (SR-SIM) of G. obscuriglobus cells with DAPI stained nucleoids (blue) and Nile Red stained membranes fed with fluorescein-labelled 40 kDa dextran (green). The nucleoid is highly condensed and multiple invaginations of the innermost membrane are visible that colocalise with the invaginated innermost membrane (Overlay). 3D reconstruction with Amira 6.0 shows dextran within the G. obscuriglobus cell (g and Supplementary Movie 3). Scale bars, 1 μm.
Figure 6. Planctopirus limnophila and Gemmata obscuriglobus possess fibres that bind to dextran.
(a–c) TEM analysis of P. limnophila cells revealed multiple distinct appendages. The flagellum (a, white arrow) with a diameter of 22 nm is typical for bacteria. In contrast, large- (a,b, white arrowheads) and small crateriform structures (a,c, asterisk) are unique to the Planctomycetes and form pili with diameters of 12 nm (b) 6 nm (c) respectively. The polar thinner fibres (a,c, asterisks) form a stalk that attaches the cell to a surface or is involved in cell aggregation. Scale bars, 0.2 μm. (d–i) Light microscopic phase contrast (d,g, Phaco) and TEM (e,f,h,i) micrographs of P. limnophila (d–f) and G. obscuriglobus cells (g–i) fed with gold-labelled dextran (four individual experiments). In the light microscope (d,g) either the stalk of P. limnophila (asterisk), that is associated with the small crateriform structures (c), or the fibres of G. obscuriglobus associated with the large crateriform structures (b, white arrowheads) are visible. TEM analysis revealed dextran binding to fibres of both species (e,f,h,i). (j) Backscattered electron detection in the SEM micrograph shows electron dense gold particles as bright dots and illustrates that fibre bundles can even be longer than the entire cell body. Some gold particles appear to be internalized by the cell (j, black arrowheads). The scheme (k) shows the flagella/attachment pole of a P. limnophila cell, which is opposite to the reproduction pole where cell division through budding takes place. While small crateriform structures are localized only close to the flagellum and produce the stalk (asterisk), fibres associated with the large crateriform structures are distributed throughout the cell (white arrowheads). Scale bars, 0.5 μm.
Similar articles
- Global and Targeted Lipid Analysis of Gemmata obscuriglobus Reveals the Presence of Lipopolysaccharide, a Signature of the Classical Gram-Negative Outer Membrane.
Mahat R, Seebart C, Basile F, Ward NL. Mahat R, et al. J Bacteriol. 2015 Oct 19;198(2):221-36. doi: 10.1128/JB.00517-15. Print 2016 Jan 15. J Bacteriol. 2015. PMID: 26483522 Free PMC article. - Bioinformatic analyses of integral membrane transport proteins encoded within the genome of the planctomycetes species, Rhodopirellula baltica.
Paparoditis P, Västermark A, Le AJ, Fuerst JA, Saier MH Jr. Paparoditis P, et al. Biochim Biophys Acta. 2014 Jan;1838(1 Pt B):193-215. doi: 10.1016/j.bbamem.2013.08.007. Epub 2013 Aug 19. Biochim Biophys Acta. 2014. PMID: 23969110 Free PMC article. - Towards understanding the molecular mechanism of the endocytosis-like process in the bacterium Gemmata obscuriglobus.
Fuerst JA, Sagulenko E. Fuerst JA, et al. Biochim Biophys Acta. 2014 Aug;1843(8):1732-8. doi: 10.1016/j.bbamcr.2013.10.002. Epub 2013 Oct 19. Biochim Biophys Acta. 2014. PMID: 24144586 Review. - Nested bacterial boxes: nuclear and other intracellular compartments in planctomycetes.
Fuerst JA, Sagulenko E. Fuerst JA, et al. J Mol Microbiol Biotechnol. 2013;23(1-2):95-103. doi: 10.1159/000346544. Epub 2013 Apr 18. J Mol Microbiol Biotechnol. 2013. PMID: 23615198 Review. - Gemmata species: Planctomycetes of medical interest.
Aghnatios R, Drancourt M. Aghnatios R, et al. Future Microbiol. 2016 May;11:659-67. doi: 10.2217/fmb-2015-0001. Epub 2016 May 9. Future Microbiol. 2016. PMID: 27158864 Review.
Cited by
- Uncovering the biotechnological capacity of marine and brackish water Planctomycetota.
Vitorino IR, Pinto E, Martín J, Mackenzie TA, Ramos MC, Sánchez P, de la Cruz M, Vicente F, Vasconcelos V, Reyes F, Lage OM. Vitorino IR, et al. Antonie Van Leeuwenhoek. 2024 Jan 23;117(1):26. doi: 10.1007/s10482-023-01923-z. Antonie Van Leeuwenhoek. 2024. PMID: 38261060 Free PMC article. - Mucisphaera calidilacus gen. nov., sp. nov., a novel planctomycete of the class Phycisphaerae isolated in the shallow sea hydrothermal system of the Lipari Islands.
Kallscheuer N, Jogler C, Peeters SH, Boedeker C, Jogler M, Heuer A, Jetten MSM, Rohde M, Wiegand S. Kallscheuer N, et al. Antonie Van Leeuwenhoek. 2022 Mar;115(3):407-420. doi: 10.1007/s10482-021-01707-3. Epub 2022 Jan 20. Antonie Van Leeuwenhoek. 2022. PMID: 35050438 Free PMC article. - Commentary: Manifold Routes to a Nucleus.
Jogler C, Wiegand S, Devos DP. Jogler C, et al. Front Microbiol. 2019 May 31;10:1198. doi: 10.3389/fmicb.2019.01198. eCollection 2019. Front Microbiol. 2019. PMID: 31214141 Free PMC article. No abstract available. - The Soil Microbiome of GLORIA Mountain Summits in the Swiss Alps.
Adamczyk M, Hagedorn F, Wipf S, Donhauser J, Vittoz P, Rixen C, Frossard A, Theurillat JP, Frey B. Adamczyk M, et al. Front Microbiol. 2019 May 15;10:1080. doi: 10.3389/fmicb.2019.01080. eCollection 2019. Front Microbiol. 2019. PMID: 31156590 Free PMC article. - Gemmata algarum, a Novel Planctomycete Isolated from an Algal Mat, Displays Antimicrobial Activity.
Kumar G, Kallscheuer N, Kashif M, Ahamad S, Jagadeeshwari U, Pannikurungottu S, Haufschild T, Kabuu M, Sasikala C, Jogler C, Ramana CV. Kumar G, et al. Mar Drugs. 2023 Dec 21;22(1):10. doi: 10.3390/md22010010. Mar Drugs. 2023. PMID: 38276648 Free PMC article.
References
- Fuerst J. A. & Sagulenko E. Beyond the bacterium: planctomycetes challenge our concepts of microbial structure and function. Nat. Rev. 9, 403–413 (2011). - PubMed
- Jeske O., Jogler M., Petersen J., Sikorski J. & Jogler C. From genome mining to phenotypic microarrays: planctomycetes as source for novel bioactive molecules. Antonie van Leeuwenhoek 104, 551–567 (2013). - PubMed
- Gimesi N. Planctomyces bekefii Gim. nov. gen. et sp. Hydrobiol. Studien 26, 1–4 (1924).
- Hirsch P. Two identical genera of budding and stalked bacteria: Planctomyces Gimesi 1924 and Blastocaulis Henrici and Johnson 1935. Int. J. Syst. Evol. Microbiol. 22, 107–111 (1972).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases