Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance - PubMed (original) (raw)
. 2017 May;23(5):556-567.
doi: 10.1038/nm.4314. Epub 2017 Apr 10.
Vishnu R Mani 1, Navyatha Mohan 1, Neha Akkad 1, Atsuo Ochi 1, Daniel W Heindel 2, Ki Buom Lee 1, Constantinos P Zambirinis 1, Gautam Sd Balasubramania Pandian 1, Shivraj Savadkar 1, Alejandro Torres-Hernandez 1, Shruti Nayak 3, Ding Wang 4, Mautin Hundeyin 1, Brian Diskin 1, Berk Aykut 1, Gregor Werba 1, Rocky M Barilla 1, Robert Rodriguez 1, Steven Chang 1, Lawrence Gardner 4, Lara K Mahal 2, Beatrix Ueberheide 3, George Miller 1 5
Affiliations
- PMID: 28394331
- PMCID: PMC5419876
- DOI: 10.1038/nm.4314
Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance
Donnele Daley et al. Nat Med. 2017 May.
Erratum in
- Author Correction: Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance.
Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, Lee KB, Zambirinis CP, Pandian GSDB, Savadkar S, Torres-Hernandez A, Nayak S, Wang D, Hundeyin M, Diskin B, Aykut B, Werba G, Barilla RM, Rodriguez R, Chang S, Gardner L, Mahal LK, Ueberheide B, Miller G. Daley D, et al. Nat Med. 2021 Dec;27(12):2247-2248. doi: 10.1038/s41591-021-01428-0. Nat Med. 2021. PMID: 34845391 No abstract available.
Abstract
The progression of pancreatic oncogenesis requires immune-suppressive inflammation in cooperation with oncogenic mutations. However, the drivers of intratumoral immune tolerance are uncertain. Dectin 1 is an innate immune receptor crucial for anti-fungal immunity, but its role in sterile inflammation and oncogenesis has not been well defined. Furthermore, non-pathogen-derived ligands for dectin 1 have not been characterized. We found that dectin 1 is highly expressed on macrophages in pancreatic ductal adenocarcinoma (PDA). Dectin 1 ligation accelerated the progression of PDA in mice, whereas deletion of Clec7a-the gene encoding dectin 1-or blockade of dectin 1 downstream signaling was protective. We found that dectin 1 can ligate the lectin galectin 9 in mouse and human PDA, which results in tolerogenic macrophage programming and adaptive immune suppression. Upon disruption of the dectin 1-galectin 9 axis, CD4+ and CD8+ T cells, which are dispensable for PDA progression in hosts with an intact signaling axis, become reprogrammed into indispensable mediators of anti-tumor immunity. These data suggest that targeting dectin 1 signaling is an attractive strategy for developing an immunotherapy for PDA.
Conflict of interest statement
Competing Financial Interests
The authors have competing interests.
GM - Co-Founder and Scientific Advisory Board Member of Nybo Therapeutics
Figures
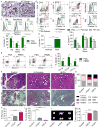
Figure 1. High Dectin-1 expression in mouse and human PDA and Dectin-1 ligation accelerates PDA progression
(a) Frozen sections of 6 month-old KC;Dectin-1+/+ and KC; Dectin-1−/− pancreata were tested for expression of Dectin-1 by IHC (scale bar = 100μm). (b) PDA-infiltrating and splenic leukocytes from KC and aged-matched WT mice were tested for expression of Dectin-1 in CD11c−Gr1−CD11b+F4/80+ macrophages, Gr1+CD11b+ neutrophils and inflammatory monocytes, and CD11c+MHCII+ dendritic cells. Representative contour plots and quantitative data from 5 mice are shown. (c) PDA-infiltrating and splenic leukocytes from mice bearing KPC-derived tumors were tested for expression of Dectin-1 compared with isotype control in CD11c−Gr1−CD11b+F4/80+ macrophages, Gr1+CD11b+ neutrophils and inflammatory monocytes, and CD11c+MHCII+ dendritic cells. Representative contour plots and quantitative data from 5 mice are shown. Murine flow cytometry experiments were repeated more than 3 times. (d) Dectin-1 expression was tested by flow cytometry in macrophages from normal pancreas compared with macrophages infiltrating orthotopic PDA tumors (n=5/group). (e) Human PDA-infiltrating and PBMC-derived CD14+, CD15+, and CD11c+ cells were tested for expression of Dectin-1. Representative contour plots (CD15, CD11c) and quantitative data from 5 PDA patients are shown. (f–i) Six week-old KC mice were treated with the Dectin-1 ligands d-Zymosan, HKCA, or vehicle for 8 weeks before sacrifice (n=5/group). (f) Representative H&E (scale bar = 100μm) and Trichrome (scale bar = 200μm) stained sections are shown. (g) The percentage of ducts exhibiting normal morphology, ADM, graded PanIN lesions, or foci of invasive cancer are shown. (h) The percentage of pancreatic area occupied by fibrotic tissues was calculated based on trichrome staining. (i) The percentage of pancreatic area occupied by normal acinar structures was calculated. (j) WT mice were administered orthotopic KPC-derived tumor cells and serially treated with the Dectin-1 ligands d-Zymosan or HKCA or vehicle control. Animals were sacrificed at 3 weeks and pancreas weights measured. Representative gross images of pancreatic tumors and quantitative data are shown (n=5/group; scale bar = 1cm; *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001).
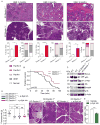
Figure 2. Dectin-1 deletion or blockade is protective against PDA
(a) KC;Dectin-1+/+ (n=10) and KC;Dectin-1−/− (n=6) mice were sacrificed at 3, 6, or 9 months of life. Representative H&E-stained sections are shown, the percentage of pancreatic area occupied by intact acinar structures, and the fractions of ductal structures exhibiting normal morphology, acino-ductal metaplasia (ADM), or graded PanIN I-III lesions were calculated (scale bar = 200μm). (b) Kaplan-Meier survival analysis was performed comparing KC;Dectin-1+/+ (n=29) and KC;Dectin-1−/− (n=41) mice (p=0.01). (c) Whole pancreas lysate from 3 month-old KC;Dectin-1+/+ and KC;Dectin-1−/− mice were assayed for expression of select oncogenic and tumor suppressor genes. (d) Six week-old KC;Dectin-1+/+ and KC;Dectin-1−/− mice were serially treated with the p-Syk inhibitor Piceatannol or vehicle for 8 weeks before sacrifice (n=5–10/group). Pancreas weights were measured and representative H&E-stained sections are shown (scale bar = 200μm). Each point represents data from a single mouse. (e) WT mice bearing orthotopic PDA were serially treated with the p-Syk inhibitor Piceatannol or vehicle for 3 weeks. Tumor-infiltrating APC were harvested and tested for p-Syk expression by flow cytometry. Median fluorescence index (MFI) is shown (n=5/group; *p<0.05; **p<0.01; ***p<0.001).
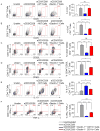
Figure 3. Dectin-1−/− PDA-infiltrating monocytic cells exhibit diminished T cell suppressive properties
Naïve CD4+ or CD8+ splenic T cells were either unstimulated, stimulated with αCD3/αCD28 alone or in co-culture with PDA-infiltrating CD11b+ cells harvested from orthotopic KPC tumors in WT or Dectin-1−/− hosts. CD4+ and CD8+ T cell activation, respectively, were determined at 72h by (a, b) ICOS expression, (c, d) the fraction of cells exhibiting the CD62L−CD44+ phenotype, (e) CD4+ T cell expression of IL-10, and (f) CD8+ T cell co-expression of IFN-γ and TNF-α. Experiments were performed in quadruplicate and repeated 3 times (*p<0.05; **p<0.01; ***p<0.001; ****p<0.0001).
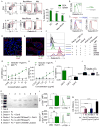
Figure 4. Dectin-1 signaling regulates macrophage infiltration and phenotype in PDA
(a–c) Six month-old KC;Dectin-1+/+ and KC;Dectin-1−/− mice were tested for (a) F4/80+ and (b) Arg1+ macrophage infiltration by IHC (n=5/group) (scale bar = 100μm). (c) The fraction of CD11c−Gr1−CD11b+F4/80+ macrophages in the pancreata of three-month old KC;Dectin-1+/+ and KC;Dectin-1−/− mice was determined by flow cytometry (n=5/group). (d–g) WT and Dectin-1−/− mice were challenged with orthotopic KPC-derived PDA tumors. (d) CD11c−Gr1−CD11b+F4/80+ macrophage infiltration was determined on day 21 by flow cytometry. (e) PDA-infiltrating macrophages were gated and tested for expression of MHC II, (f) CD206, (g) TNF-α, and iNOS (n=5/group). (h–k) WT mice were implanted with orthotopic KPC tumors and serially treated with the Dectin-1 specific ligand d-Zymosan or vehicle. (h) Dectin-1 activation was confirmed by upregulation of p-Syk expression in PDA-infiltrating macrophages in d-Zymosan-treated compared with vehicle-treated mice. (i) The fraction of tumor-infiltrating macrophages was determined by flow cytometry on day 21. (j) PDA-infiltrating macrophages were gated and tested for expression of CD206 and (k) MHC II. Representative contour plots and quantitative data are shown (n=5/group). Experiments were repeated more than 3 times with similar results. (l) Dectin-1−/− mice were subcutaneously implanted with KPC-derived PDA tumor cells admixed with WT or Dectin-1−/− macrophages. Tumor volume was recorded at serial intervals. This experiment was repeated twice with similar results (n=4/group; *p<0.05; **p<0.01; ****p<0.0001).
Figure 5. Dectin-1 signaling prevents immunogenic T cell differentiation in PDA
(a–d) The inferior pancreas-draining lymph node in 6 month-old KC;Dectin-1+/+ and KC;Dectin-1−/− mice was assayed for (a) CD44, (b) Ox40, and (c) PD-1 expression in CD4+ and CD8+ T cells, and (d) the CD8+:CD4+ T cell ratio. Representative contour plots and quantitative data are shown (n=5/group). (e–g) WT and Dectin-1−/− mice were challenged with orthotopic KPC tumors. (e) The CD8+:CD4+ ratio was determined on day 21 by flow cytometry. (f) PDA-infiltrating CD8+ T cell expression of PD-1, T-bet, TNF-α, CD107a, and Granzyme B were determined by flow cytometry. (g) PDA-infiltrating CD4+ T cell expression of CD44, CD107a, ICOS, T-bet, and TNF-α were determined by flow cytometry (n=5/group). (h, i) WT and Dectin-1−/− mice were challenged with orthotopic KPC tumors. Cohorts were additionally treated with αPD-1 or isotype control. (h) Tumor weights were measured on Day 21 and (i) the fraction of CD4+ T cells expressing IFN-γ was determined by flow cytometry n=5/group. All experiments were repeated at least twice (*p<0.05, **p<0.01, ***p<0.001).
Figure 6. Galectin-9 is a novel Dectin-1 ligand in PDA
(a) PDA-infiltrating and splenic Gr1+CD11b+ neutrophils and inflammatory monocytes, CD11c−Gr1−CD11b+F4/80+ macrophages, and CD11c+MHCII+ DC from mice harboring orthotopic KPC tumors were gated by flow cytometry and tested for expression of Galectin-9. Gates are based on respective isotype control (not shown). Representative contour plots and quantitative data from 5 mice are shown. (b) CD45−CD133+ pancreatic cancer cells from orthotopic KPC tumors were gated by flow cytometry and tested for expression of Galectin-9 compared with isotype control. Representative data from >3 experiments is shown. (c) CD45+ and CD45− cells from human PDA tumor tissue were tested for expression of Galectin-9 compared with PBMC. Representative data from one of three patients are shown. (d) Frozen sections of orthotopic KPC-derived pancreatic tumors were co-stained for CD45 and Galectin-9 or isotype control and imaged by confocal microscopy. Representative images are shown (scale bar = 50μm). (e) Frozen sections of orthotopic KPC-derived pancreatic tumors were co-stained for CK19 and Galectin-9 or isotype control and imaged by confocal microscopy. Representative images are shown (scale bar = 50μm). (f) Protein G-magnetic beads were loaded with the Dectin-1 IgG Fc fusion protein. After blocking, the bead–IgG Fc complexes were incubated with recombinant Galectin-9 and then stained with fluorescently-conjugated anti-Galectin-9 and tested for fluorescence by flow cytometry. Controls included: unstained beads, bead–IgG Fc complexes + recombinant Galectin-9 + fluorescently-conjugated isotype antibody, bead–IgG Fc complexes + fluorescently-conjugated anti-Galectin-9, beads without Dectin-1 IgG Fc incubated with recombinant Galectin-9 + fluorescently-conjugated anti-Galectin-9. To test for competitive inhibition of Galectin-9 binding with a well-characterized Dectin-1 ligand, the bead–IgG Fc complexes were incubated with recombinant Galectin-9 together with d-Zymosan and then stained with fluorescently-conjugated anti-Galectin-9. This assay was repeated twice with similar results. (g, h) Galectin-9 coated ELISA plates were incubated with increasing doses of (g) murine or (h) human Dectin-1 IgG Fc or control IgG Fc. The Galectin-9-bound Dectin-1 IgG Fc was detected with anti-IgG-HRP. Averages of triplicates are shown. ELISA assays was repeated twice with similar results. (i) Galectin-3, Galectin-4, and Galectin-9 coated ELISA plates were incubated with Dectin-1 IgG Fc or control IgG Fc (2.5μg/ml) in parallel. The Galectin-bound Dectin-1 IgG Fc was detected with anti-IgG-HRP. Averages of triplicates are shown. (j) We precipitated Dectin-1 ligands in pancreatic tissue extract from 6 month-old KC mice using the Dectin-1 IgG Fc or control IgG Fc and then probed for Galectin-9 by western blotting. Recombinant Galectin-9 was used as a positive control. This assay was repeated twice with similar results. (k) Dectin-1 IgG Fc was treated with either buffer or PNGase F, loaded onto Protein G beads and incubated with recombinant mouse Galectin-9 pre-incubated with 100 mM lactose or buffer. Treated and control samples were analyzed by SDS-PAGE and gels were stained with Coomassie Blue. This experiment was repeated twice with similar results. (l) WT and Dectin-1−/− macrophages were treated with Galectin-9 (10ug/ml) for 3 hours. Syk phosphorylation was determined by flow cytometry compared with isotype control. Representative histogram overlays and quantitative data from 5 separate experiments are shown. (m) Dectin-1 reporter HEK293 cells were untreated or treated low and high doses of Galectin-9 or well-characterized Dectin-1 ligands Curdlan and d-Zymosan. Dectin-1 activation was measured by detection of secreted embryonic alkaline phosphatase. This assay was performed in triplicate (*p<0.05; **p<0.01; ****p<0.0001).
Similar articles
- The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression.
Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, Torres-Hernandez A, Pergamo M, Ochi A, Zambirinis CP, Pansari M, Rendon M, Tippens D, Hundeyin M, Mani VR, Hajdu C, Engle D, Miller G. Seifert L, et al. Nature. 2016 Apr 14;532(7598):245-9. doi: 10.1038/nature17403. Epub 2016 Apr 6. Nature. 2016. PMID: 27049944 Free PMC article. - Tumor Cell-Derived IL1β Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer.
Das S, Shapiro B, Vucic EA, Vogt S, Bar-Sagi D. Das S, et al. Cancer Res. 2020 Mar 1;80(5):1088-1101. doi: 10.1158/0008-5472.CAN-19-2080. Epub 2020 Jan 8. Cancer Res. 2020. PMID: 31915130 Free PMC article. - The receptor for advanced glycation end products promotes pancreatic carcinogenesis and accumulation of myeloid-derived suppressor cells.
Vernon PJ, Loux TJ, Schapiro NE, Kang R, Muthuswamy R, Kalinski P, Tang D, Lotze MT, Zeh HJ 3rd. Vernon PJ, et al. J Immunol. 2013 Feb 1;190(3):1372-9. doi: 10.4049/jimmunol.1201151. Epub 2012 Dec 26. J Immunol. 2013. PMID: 23269246 Free PMC article. - Challenges and Opportunities for Pancreatic Cancer Immunotherapy.
Bear AS, Vonderheide RH, O'Hara MH. Bear AS, et al. Cancer Cell. 2020 Dec 14;38(6):788-802. doi: 10.1016/j.ccell.2020.08.004. Epub 2020 Sep 17. Cancer Cell. 2020. PMID: 32946773 Free PMC article. Review. - Novel targets in pancreatic cancer research.
Kozak G, Blanco FF, Brody JR. Kozak G, et al. Semin Oncol. 2015 Feb;42(1):177-87. doi: 10.1053/j.seminoncol.2014.12.015. Epub 2014 Dec 10. Semin Oncol. 2015. PMID: 25726061 Review.
Cited by
- The regulating role of galectin-9 in immune cell populations.
Cao Z, Leng P, Xu H, Li X. Cao Z, et al. Front Pharmacol. 2024 Oct 30;15:1462061. doi: 10.3389/fphar.2024.1462061. eCollection 2024. Front Pharmacol. 2024. PMID: 39539619 Free PMC article. Review. - Pancreatic cancer-derived exosomal miR-510 promotes macrophage M2 polarization and facilitates cancer cell aggressive phenotypes.
Wang T, Ye L, Zhou Y, Zhang X, Li R, Zhou Y, Weng J, Mo Q, Yu Y. Wang T, et al. Hum Cell. 2024 Nov 12;38(1):17. doi: 10.1007/s13577-024-01144-0. Hum Cell. 2024. PMID: 39528705 - The Dectin-1 and Dectin-2 clusters: C-type lectin receptors with fundamental roles in immunity.
Malamud M, Brown GD. Malamud M, et al. EMBO Rep. 2024 Oct 31. doi: 10.1038/s44319-024-00296-2. Online ahead of print. EMBO Rep. 2024. PMID: 39482490 Review. - Galectin-9 - ligand axis: an emerging therapeutic target for multiple myeloma.
Shil RK, Mohammed NBB, Dimitroff CJ. Shil RK, et al. Front Immunol. 2024 Sep 25;15:1469794. doi: 10.3389/fimmu.2024.1469794. eCollection 2024. Front Immunol. 2024. PMID: 39386209 Free PMC article. Review. - Integrative analyses of bulk and single-cell RNA-seq reveals the correlation between SPP1+ macrophages and resistance to neoadjuvant chemoimmunotherapy in esophageal squamous cell carcinoma.
Geng Z, Li F, Yang Z, Li B, Xu Y, Wu B, Sheng Y, Yuan P, Huang L, Qi Y. Geng Z, et al. Cancer Immunol Immunother. 2024 Oct 5;73(12):257. doi: 10.1007/s00262-024-03848-6. Cancer Immunol Immunother. 2024. PMID: 39367943 Free PMC article.
References
- Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer cell. 2007;11:291–302. - PubMed
MeSH terms
Substances
Grants and funding
- UL1 TR001445/TR/NCATS NIH HHS/United States
- P30 CA016087/CA/NCI NIH HHS/United States
- R01 CA168611/CA/NCI NIH HHS/United States
- T32 CA193111/CA/NCI NIH HHS/United States
- R21 CA155649/CA/NCI NIH HHS/United States
- R01 CA206105/CA/NCI NIH HHS/United States
- K08 DK085278/DK/NIDDK NIH HHS/United States
- UL1 TR000038/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials