Comparison of ePlex Respiratory Pathogen Panel with Laboratory-Developed Real-Time PCR Assays for Detection of Respiratory Pathogens - PubMed (original) (raw)
Comparison of ePlex Respiratory Pathogen Panel with Laboratory-Developed Real-Time PCR Assays for Detection of Respiratory Pathogens
R H T Nijhuis et al. J Clin Microbiol. 2017 Jun.
Abstract
Infections of the respiratory tract can be caused by a diversity of pathogens, both viral and bacterial. Rapid microbiological diagnosis ensures appropriate antimicrobial therapy as well as effective implementation of isolation precautions. The ePlex respiratory pathogen panel (RP panel) is a novel molecular biology-based assay, developed by GenMark Diagnostics, Inc. (Carlsbad, CA), to be performed within a single cartridge for the diagnosis of 25 respiratory pathogens (viral and bacterial). The objective of this study was to compare the performance of the RP panel with those of laboratory-developed real-time PCR assays, using a variety of previously collected clinical respiratory specimens. A total of 343 clinical specimens as well as 29 external quality assessment (EQA) specimens and 2 different Middle East respiratory syndrome coronavirus isolates have been assessed in this study. The RP panel showed an agreement of 97.4% with the real-time PCR assay regarding 464 pathogens found in the clinical specimens. All pathogens present in clinical samples and EQA samples with a threshold cycle (CT ) value of <30 were detected correctly using the RP panel. The RP panel detected 17 additional pathogens, 7 of which could be confirmed by discrepant testing. In conclusion, this study shows excellent performance of the RP panel in comparison to real-time PCR assays for the detection of respiratory pathogens. The ePlex system provided a large amount of useful diagnostic data within a short time frame, with minimal hands-on time, and can therefore potentially be used for rapid diagnostic sample-to-answer testing, in either a laboratory or a decentralized setting.
Keywords: influenza; point-of-care testing; rapid diagnostics; respiratory pathogens; sample-to-answer.
Copyright © 2017 Nijhuis et al.
Figures
FIG 1
ePlex system (A) and the corresponding cartridge of the ePlex respiratory pathogen panel (B).
FIG 2
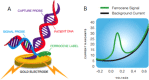
Principle of the eSensor detection technology. Amplified sequences of the targeted pathogens are detected electrochemically using a complementary pathogen-specific signal probe tagged with ferrocene, a reducing agent. The hybridized molecule is then exposed to another sequence-specific probe that is bound to a solid phase, which is a gold electrode (A). Upon binding of the two molecules, the ferrocene comes in close proximity to the gold electrode, where an electron transfer that can be measured using GenMark's eSensor technology on the ePlex system can occur (B).
FIG 3
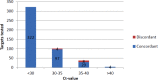
Pathogen concordance by CT value.
Similar articles
- The GenMark ePlex®: another weapon in the syndromic arsenal for infection diagnosis.
Schmitz JE, Tang YW. Schmitz JE, et al. Future Microbiol. 2018 Dec;13(16):1697-1708. doi: 10.2217/fmb-2018-0258. Epub 2018 Dec 14. Future Microbiol. 2018. PMID: 30547684 Free PMC article. Review. - Clinical implications of rapid ePlex® Respiratory Pathogen Panel testing compared to laboratory-developed real-time PCR.
van Rijn AL, Nijhuis RHT, Bekker V, Groeneveld GH, Wessels E, Feltkamp MCW, Claas ECJ. van Rijn AL, et al. Eur J Clin Microbiol Infect Dis. 2018 Mar;37(3):571-577. doi: 10.1007/s10096-017-3151-0. Epub 2017 Dec 8. Eur J Clin Microbiol Infect Dis. 2018. PMID: 29222697 Free PMC article. - Multicenter Evaluation of the ePlex Respiratory Pathogen Panel for the Detection of Viral and Bacterial Respiratory Tract Pathogens in Nasopharyngeal Swabs.
Babady NE, England MR, Jurcic Smith KL, He T, Wijetunge DS, Tang YW, Chamberland RR, Menegus M, Swierkosz EM, Jerris RC, Greene W. Babady NE, et al. J Clin Microbiol. 2018 Jan 24;56(2):e01658-17. doi: 10.1128/JCM.01658-17. Print 2018 Feb. J Clin Microbiol. 2018. PMID: 29212701 Free PMC article. - Comparison of the FilmArray Respiratory Panel and Prodesse real-time PCR assays for detection of respiratory pathogens.
Loeffelholz MJ, Pong DL, Pyles RB, Xiong Y, Miller AL, Bufton KK, Chonmaitree T. Loeffelholz MJ, et al. J Clin Microbiol. 2011 Dec;49(12):4083-8. doi: 10.1128/JCM.05010-11. Epub 2011 Oct 12. J Clin Microbiol. 2011. PMID: 21998418 Free PMC article. - Multiplex PCR and emerging technologies for the detection of respiratory pathogens.
Caliendo AM. Caliendo AM. Clin Infect Dis. 2011 May;52 Suppl 4(Suppl 4):S326-30. doi: 10.1093/cid/cir047. Clin Infect Dis. 2011. PMID: 21460291 Free PMC article. Review.
Cited by
- Comparing the Cobas Liat Influenza A/B and respiratory syncytial virus assay with multiplex nucleic acid testing.
Gosert R, Naegele K, Hirsch HH. Gosert R, et al. J Med Virol. 2019 Apr;91(4):582-587. doi: 10.1002/jmv.25344. Epub 2018 Nov 13. J Med Virol. 2019. PMID: 30345524 Free PMC article. - Developmental roadmap for antimicrobial susceptibility testing systems.
van Belkum A, Bachmann TT, Lüdke G, Lisby JG, Kahlmeter G, Mohess A, Becker K, Hays JP, Woodford N, Mitsakakis K, Moran-Gilad J, Vila J, Peter H, Rex JH, Dunne WM Jr; JPIAMR AMR-RDT Working Group on Antimicrobial Resistance and Rapid Diagnostic Testing. van Belkum A, et al. Nat Rev Microbiol. 2019 Jan;17(1):51-62. doi: 10.1038/s41579-018-0098-9. Nat Rev Microbiol. 2019. PMID: 30333569 Free PMC article. Review. - Influenza species and subtypes circulation among hospitalized patients in Laleh hospital during two influenza seasonal (2016-2017 and 2017-2018) using a multiplex Real Time-Polymerase Chain Reaction.
Azhar IR, Mohraz M, Mardani M, Tavakoli MA, Afshar AE, Zamani M, Sadeghpoor S, Safari S, Dadashpoor R, Rezaee M, Shirvani F, Azimi S, Heydarifard Z, Ranjbar HH, Lotfi AH, Mosadegh F, Hashemnejad F, Jazayeri SM. Azhar IR, et al. Infect Dis Rep. 2020 Apr 15;12(1):8139. doi: 10.4081/idr.2020.8139. eCollection 2020 Feb 25. Infect Dis Rep. 2020. PMID: 32318254 Free PMC article. - Combined SARS-CoV-2 nucleic acid amplification testing and respiratory virus panel RT-PCR on the Hologic Panther Fusion system.
Stevens BA, Hogan CA, Mfuh KO, Khan G, Sahoo MK, Huang C, Garamani N, Zehnder J, Kurzer J, Pinsky BA. Stevens BA, et al. J Clin Virol. 2021 May;138:104792. doi: 10.1016/j.jcv.2021.104792. Epub 2021 Mar 10. J Clin Virol. 2021. PMID: 33770659 Free PMC article. - The GenMark ePlex®: another weapon in the syndromic arsenal for infection diagnosis.
Schmitz JE, Tang YW. Schmitz JE, et al. Future Microbiol. 2018 Dec;13(16):1697-1708. doi: 10.2217/fmb-2018-0258. Epub 2018 Dec 14. Future Microbiol. 2018. PMID: 30547684 Free PMC article. Review.
References
- Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. 2010. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 48:2940–2947. doi:10.1128/JCM.00636-10. - DOI - PMC - PubMed
- Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. 2004. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza a and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol 42:1564–1569. doi:10.1128/JCM.42.4.1564-1569.2004. - DOI - PMC - PubMed
- Templeton KE, Scheltinga SA, Graffelman AW, Van Schie JM, Crielaard JW, Sillekens P, Van Den Broek PJ, Goossens H, Beersma MF, Claas EC. 2003. Comparison and evaluation of real-time PCR, real-time nucleic acid sequence-based amplification, conventional PCR, and serology for diagnosis of Mycoplasma pneumoniae. J Clin Microbiol 41:4366–4371. doi:10.1128/JCM.41.9.4366-4371.2003. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical