Lipid Nanoparticle Systems for Enabling Gene Therapies - PubMed (original) (raw)
Review
Lipid Nanoparticle Systems for Enabling Gene Therapies
Pieter R Cullis et al. Mol Ther. 2017.
Abstract
Genetic drugs such as small interfering RNA (siRNA), mRNA, or plasmid DNA provide potential gene therapies to treat most diseases by silencing pathological genes, expressing therapeutic proteins, or through gene-editing applications. In order for genetic drugs to be used clinically, however, sophisticated delivery systems are required. Lipid nanoparticle (LNP) systems are currently the lead non-viral delivery systems for enabling the clinical potential of genetic drugs. Application will be made to the Food and Drug Administration (FDA) in 2017 for approval of an LNP siRNA drug to treat transthyretin-induced amyloidosis, presently an untreatable disease. Here, we first review research leading to the development of LNP siRNA systems capable of silencing target genes in hepatocytes following systemic administration. Subsequently, progress made to extend LNP technology to mRNA and plasmids for protein replacement, vaccine, and gene-editing applications is summarized. Finally, we address current limitations of LNP technology as applied to genetic drugs and ways in which such limitations may be overcome. It is concluded that LNP technology, by virtue of robust and efficient formulation processes, as well as advantages in potency, payload, and design flexibility, will be a dominant non-viral technology to enable the enormous potential of gene therapy.
Keywords: gene editing; gene therapy; genetic drugs; lipid nanoparticles; mRNA; siRNA.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures
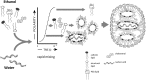
Figure 1
Ethanol Loading Formulation Process for LNP Containing Oligonucleotides Such as siRNA Lipids dissolved in ethanol are rapidly mixed with oligonucleotides in aqueous buffer (pH 4). The head groups of the ionizable cationic lipids are indicated as blue, of the PEG-lipids as white lines and of DSPC as white circles. Cholesterol is indicated as a diamond orange symbol and acyl chains as yellow lines. On mixing, electrostatic interactions drive formation of an inverted micelle containing oligonucleotide (red zig-zag symbol) surrounded by predominantly cationic lipid that falls out of solution as the polarity is raised. If the mixing process occurs rapidly enough, these inverted micelles do not have time to aggregate but rather are coated with PEG-lipid, which precipitates at higher polarity to surround the inverted micelles.
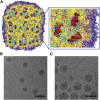
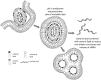
Figure 2
Structure of LNP-Oligonucleotide Systems (A) Molecular modeling indicates that LNP-nucleic acid systems contain irregular water-filled cavities surrounded by lipid monolayers, with nucleic acids bound to monolayer surfaces: cross-section and zoom views. Yellow, cationic lipid; pink, cholesterol; gray, DSPC; cyan, lipid polar moiety; violet, PEG-lipid; red, nucleic acids (duplex DNA); water not shown for clarity. The lipid composition was cationic lipid/DSPC/cholesterol/PEG-lipid (4:1:4:1; mol/mol) and DNA-to-lipid ratio ∼0.05 wt/wt. Adapted from Leung et al. (B) Lipid nanoparticle systems containing siRNA exhibit “solid core” morphology as visualized by cryo-TEM microscopy. Cryo-TEM micrograph obtained from LNP siRNA with lipid composition DLinKC2-DMA/DSPC/Chol/PEG-lipid (40/11.5/47.5/1; mol/mol) and siRNA at a siRNA/lipid ratio of 0.06, wt/wt, corresponding to an siRNA/cationic lipid charge ratio of 0.25. Scale bar, 100 nm. (C) LNP containing gold nanoparticles (5 nm diameter) exhibit a “currant bun” morphology. LNP encapsulating negatively charged gold nanoparticles (5 nm diameter) prepared with DLin-KC2-DMA/DOPE/Chol/PEG-lipid (40/11.5/47.5/1; % mol) at an Au-NP-to-lipid ratio of 2.2 × 1013 particles/μmol lipid. Scale bar, 100 nm.
Figure 3
Evolution of Ionizable Cationic Lipids DODAP, originally used for encapsulating antisense oligonucleotides, was replaced by DLinDMA in LNP siRNA systems to enhance potency. Subsequently DLinKC2DMA was found to further enhance potency, leading to a large synthesis effort to arrive at the gold standard ionizable cationic lipid DLinMC3DMA.
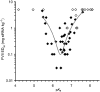
Figure 4
Optimized Ionizable Cationic Lipids Exhibit pKa Values in the Range pKa = 6.2–6.5 The figure presents a plot of the dose required to achieve 50% gene silencing (ED50) in hepatocytes in mice versus the pKa of the cationic lipids employed in the LNP. Fifty-six amino lipids were synthesized and formulated in LNPs and the ED50 measured and plotted against their pKa. For 15 lipids the ED50 dose was not achieved, these are indicated by the open diamonds representing the highest dose tested for that lipid. For the remaining lipids (closed diamonds), a polynomial best-fit curve highlights the most active compounds, which exhibit an optimal pKa between 6.2 and 6.6. Each data point is derived from a four-dose response curve with groups of n = 4 mice per dose. Reproduced with permission from Jayaraman et al.
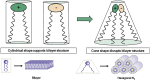
Figure 5
Proposed Mechanism of Action for Membrane Disruptive Effects of Cationic Lipids In isolation, cationic lipids and anionic lipids present in the endosome (such as lyso-bis phosphatidic acid) adopt a cylindrical molecular shape, which is compatible with packing in a bilayer configuration. However, when cationic and anionic lipids are mixed together, they combine to form ion pairs where the cross-sectional area of the combined head group is less than that of the sum of individual head group areas in isolation. The ion pair therefore adopts a molecular “cone” shape, which promotes the formation of inverted, non-bilayer phases such as the hexagonal HII phase illustrated. Inverted phases do not support bilayer structure and are associated with membrane fusion and membrane disruption. Adapted from Semple et al.
Figure 6
Proposed Mechanism Whereby LNP Containing Ionizable Cationic Lipids and Genetic Drugs Deliver Encapsulated RNA or DNA to the Cell Cytoplasm The LNP is accumulated into target cells by endocytosis following adsorption of ApoE. As the pH of the endosome is reduced by inwardly directed proton pumps the ionizable cationic lipid becomes progressively protonated (positively charged). Endogenous anionic lipids displace the cationic lipids from the genetic drug and combine with the cationic lipids to produce non-bilayer structures resulting in disruption of the endosomal membrane and release of the genetic drug into the cell cytoplasm.
Similar articles
- Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility.
Kulkarni JA, Cullis PR, van der Meel R. Kulkarni JA, et al. Nucleic Acid Ther. 2018 Jun;28(3):146-157. doi: 10.1089/nat.2018.0721. Epub 2018 Apr 23. Nucleic Acid Ther. 2018. PMID: 29683383 Review. - Lipid Nanoparticle Technology for Clinical Translation of siRNA Therapeutics.
Kulkarni JA, Witzigmann D, Chen S, Cullis PR, van der Meel R. Kulkarni JA, et al. Acc Chem Res. 2019 Sep 17;52(9):2435-2444. doi: 10.1021/acs.accounts.9b00368. Epub 2019 Aug 9. Acc Chem Res. 2019. PMID: 31397996 Review. - Development of lipid nanoparticle formulations of siRNA for hepatocyte gene silencing following subcutaneous administration.
Chen S, Tam YY, Lin PJ, Leung AK, Tam YK, Cullis PR. Chen S, et al. J Control Release. 2014 Dec 28;196:106-12. doi: 10.1016/j.jconrel.2014.09.025. Epub 2014 Oct 5. J Control Release. 2014. PMID: 25285610 - Lipid nanoparticles for short interfering RNA delivery.
Leung AK, Tam YY, Cullis PR. Leung AK, et al. Adv Genet. 2014;88:71-110. doi: 10.1016/B978-0-12-800148-6.00004-3. Adv Genet. 2014. PMID: 25409604 Free PMC article. Review. - Cationic lipid nanoparticles for therapeutic delivery of siRNA and miRNA to murine liver tumor.
Hsu SH, Yu B, Wang X, Lu Y, Schmidt CR, Lee RJ, Lee LJ, Jacob ST, Ghoshal K. Hsu SH, et al. Nanomedicine. 2013 Nov;9(8):1169-80. doi: 10.1016/j.nano.2013.05.007. Epub 2013 May 30. Nanomedicine. 2013. PMID: 23727126 Free PMC article.
Cited by
- mRNA vaccines for COVID-19: what, why and how.
Park JW, Lagniton PNP, Liu Y, Xu RH. Park JW, et al. Int J Biol Sci. 2021 Apr 10;17(6):1446-1460. doi: 10.7150/ijbs.59233. eCollection 2021. Int J Biol Sci. 2021. PMID: 33907508 Free PMC article. Review. - Advances in nucleic acid therapeutics: structures, delivery systems, and future perspectives in cancer treatment.
Zhang L, Lou W, Wang J. Zhang L, et al. Clin Exp Med. 2024 Aug 28;24(1):200. doi: 10.1007/s10238-024-01463-4. Clin Exp Med. 2024. PMID: 39196428 Free PMC article. Review. - Lipid-based nucleic acid therapeutics with in vivo efficacy.
Sufian MA, Ilies MA. Sufian MA, et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023 Mar;15(2):e1856. doi: 10.1002/wnan.1856. Epub 2022 Sep 30. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023. PMID: 36180107 Free PMC article. Review. - Nanoformulations targeting immune cells for cancer therapy: mRNA therapeutics.
Yang W, Cao J, Cheng H, Chen L, Yu M, Chen Y, Cui X. Yang W, et al. Bioact Mater. 2023 May;23:438-470. doi: 10.1016/j.bioactmat.2022.11.014. Epub 2022 Dec 1. Bioact Mater. 2023. PMID: 36471724 Free PMC article. - Tumor-tropic Trojan horses: Using mesenchymal stem cells as cellular nanotheranostics.
Rosu A, Ghaemi B, Bulte JWM, Shakeri-Zadeh A. Rosu A, et al. Theranostics. 2024 Jan 1;14(2):571-591. doi: 10.7150/thno.90187. eCollection 2024. Theranostics. 2024. PMID: 38169524 Free PMC article. Review.
References
- Cullis P.R., Mayer L.D., Bally M.B., Madden T.D., Hope M.J. Generating and loading of liposomal systems for drug-delivery applications. Adv. Drug Deliv. Rev. 1989;3:267–282.
- Cullis P.R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta. 1979;559:399–420. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials