Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage - PubMed (original) (raw)
Review
Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage
Scott S Shafiei et al. Front Aging Neurosci. 2017.
Abstract
Aging has long been considered as the main risk factor for several neurodegenerative disorders including a large group of diseases known as tauopathies. Even though neurofibrillary tangles (NFTs) have been examined as the main histopathological hallmark, they do not seem to play a role as the toxic entities leading to disease. Recent studies suggest that an intermediate form of tau, prior to NFT formation, the tau oligomer, is the true toxic species. However, the mechanisms by which tau oligomers trigger neurodegeneration remain unknown. This review summarizes recent findings regarding the role of tau oligomers in disease, including release from cells, propagation from affected to unaffected brain regions, uptake into cells, and toxicity via mitochondrial dysfunction. A greater understanding of tauopathies may lead to future advancements in regards to prevention and treatment.
Keywords: mitochondrial dysfunction; propagation; tau oligomers; tau secretion; tauopathy.
Figures
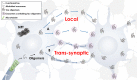
Figure 1
Propagation of tau oligomers. Schematic representation of free or exosomal tau oligomers release, local or trans-synaptic. In local transmission, tau oligomers are released from one neuron and taken up by another neuron in the vicinity. In trans-synaptic transmission, tau oligomers and/or exosomes containing tau oligomers are passed across the synapse of two neighboring neurons.
Figure 2
Mechanisms of tau oligomer internalization and mitochondrial damage. Schematic representation of the proposed mechanisms contributing to tau oligomer internalization that may lead to mitochondrial dysfunction and cell death. Methods of tau oligomer internalization include (1) dynamin-driven endocytosis; (2) muscarinic (M1 and M3) receptor-mediated endocytosis; (3) proteoglycan-mediated macropinocytosis (HSPGs); (4) exosomes; and (5) annular protofibrils. Internalized tau oligomers interfere with the mitochondrial respiratory chain, inducing cytochrome c release and stimulating reactive oxygen species (ROS) production. Tau oligomers induce mitochondrial fusion/fission imbalance by binding with dynamin-related protein 1 (DRP1). Tau oligomers interact with the outer membrane porin protein.
Similar articles
- Formation and propagation of tau oligomeric seeds.
Gerson JE, Kayed R. Gerson JE, et al. Front Neurol. 2013 Jul 17;4:93. doi: 10.3389/fneur.2013.00093. eCollection 2013. Front Neurol. 2013. PMID: 23882255 Free PMC article. - Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds.
Castillo-Carranza DL, Gerson JE, Sengupta U, Guerrero-Muñoz MJ, Lasagna-Reeves CA, Kayed R. Castillo-Carranza DL, et al. J Alzheimers Dis. 2014;40 Suppl 1:S97-S111. doi: 10.3233/JAD-132477. J Alzheimers Dis. 2014. PMID: 24603946 - Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer's disease.
Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A. Maeda S, et al. Neurosci Res. 2006 Mar;54(3):197-201. doi: 10.1016/j.neures.2005.11.009. Epub 2006 Jan 6. Neurosci Res. 2006. PMID: 16406150 - The prion-like transmission of tau oligomers via exosomes.
Jackson NA, Guerrero-Muñoz MJ, Castillo-Carranza DL. Jackson NA, et al. Front Aging Neurosci. 2022 Aug 18;14:974414. doi: 10.3389/fnagi.2022.974414. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36062141 Free PMC article. Review. - The role of tau oligomers in the onset of Alzheimer's disease neuropathology.
Cárdenas-Aguayo Mdel C, Gómez-Virgilio L, DeRosa S, Meraz-Ríos MA. Cárdenas-Aguayo Mdel C, et al. ACS Chem Neurosci. 2014 Dec 17;5(12):1178-91. doi: 10.1021/cn500148z. Epub 2014 Oct 15. ACS Chem Neurosci. 2014. PMID: 25268947 Review.
Cited by
- Normal and Pathological Tau Uptake Mediated by M1/M3 Muscarinic Receptors Promotes Opposite Neuronal Changes.
Morozova V, Cohen LS, Makki AE, Shur A, Pilar G, El Idrissi A, Alonso AD. Morozova V, et al. Front Cell Neurosci. 2019 Sep 4;13:403. doi: 10.3389/fncel.2019.00403. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31555098 Free PMC article. - Investigation of the Structure of Full-Length Tau Proteins with Coarse-Grained and All-Atom Molecular Dynamics Simulations.
He X, Man VH, Gao J, Wang J. He X, et al. ACS Chem Neurosci. 2023 Jan 18;14(2):209-217. doi: 10.1021/acschemneuro.2c00381. Epub 2022 Dec 23. ACS Chem Neurosci. 2023. PMID: 36563129 Free PMC article. - In Vivo Validation of a Small Molecule Inhibitor of Tau Self-Association in htau Mice.
Davidowitz EJ, Krishnamurthy PK, Lopez P, Jimenez H, Adrien L, Davies P, Moe JG. Davidowitz EJ, et al. J Alzheimers Dis. 2020;73(1):147-161. doi: 10.3233/JAD-190465. J Alzheimers Dis. 2020. PMID: 31771053 Free PMC article. - Zn2+ Aggravates Tau Aggregation and Neurotoxicity.
Li X, Du X, Ni J. Li X, et al. Int J Mol Sci. 2019 Jan 23;20(3):487. doi: 10.3390/ijms20030487. Int J Mol Sci. 2019. PMID: 30678122 Free PMC article. - The γ-Adducin 1-357 fragment promotes tau pathology.
Yu H, Xiong M, Liu C, Xia D, Meng L, Zhang Z. Yu H, et al. Front Aging Neurosci. 2023 Sep 13;15:1241750. doi: 10.3389/fnagi.2023.1241750. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37771520 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources