Comparative effectiveness of glycemic control in patients with type 2 diabetes treated with GLP-1 receptor agonists: a network meta-analysis of placebo-controlled and active-comparator trials - PubMed (original) (raw)
Comparative effectiveness of glycemic control in patients with type 2 diabetes treated with GLP-1 receptor agonists: a network meta-analysis of placebo-controlled and active-comparator trials
Michelle E Orme et al. Diabetes Metab Syndr Obes. 2017.
Abstract
Background: Clinical studies of patients with type 2 diabetes show that GLP-1 receptor agonists (GLP-1 RAs) improve glycemic control and promote weight loss. We conducted a Bayesian network meta-analysis (NMA) of placebo- and active-controlled randomized trials to assess the comparative effectiveness of liraglutide, albiglutide, dulaglutide, and exenatide twice daily and once weekly, with a focus on glycemic control.
Materials and methods: We searched Medline, Embase, and the Cochrane Library (up to December 2014) for core registration programs for US-approved GLP-1 RAs. Patients reaching an A1C target of <7% were analyzed with a binomial model and change in A1C from baseline with a normal model. A covariate analysis assessed the impact of baseline A1C and treatment background on outcomes.
Results: The base-case NMA used 23 trials reporting A1C outcomes at ~6 month follow-up. The results, unadjusted and adjusted for baseline A1C, indicated that all GLP-1 RAs resulted in statistically significantly lower A1C at follow-up compared with placebo. The odds of reaching the <7% target were also significantly better compared with placebo. With dulaglutide, exenatide once weekly, and liraglutide, the absolute reduction in A1C at 6 months was 0.9%-1.4%, and was significantly better than exenatide twice daily. Albiglutide was not significantly different from exenatide twice daily. We estimate that ~50% of patients will meet the <7% A1C target within 6 months of commencing GLP-1 RAs.
Conclusion: This was a comprehensive assessment of the comparative effectiveness of GLP-1 RAs and A1C outcome. GLP-1 RAs are a viable addition to oral antidiabetes therapy, and dulaglutide, exenatide once weekly, and liraglutide are the most effective.
Keywords: GLP-1 RAs; comparative effectiveness; glucagon-like peptide-1-receptor agonists; network meta-analysis; type 2 diabetes.
Conflict of interest statement
Disclosure MEO received funding from AstraZeneca US, and HN, JL, and SAT are paid employees of AstraZeneca US. The authors report no other conflicts of interest in this work.
Figures
Figure 1
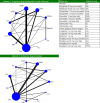
Network diagram for meta-analysis of A1C outcomes. Notes: Network 2 includes all 29 studies, network 1 (the base case) excludes LEAD-3 and HARMONY 1–5– (23 studies); line thickness corresponds to number of study arms contributing to analysis. Abbreviations: EBID, exenatide bis in die (twice daily); EQW, exenatide quaque week (once weekly); Dula, dulaglutide; Ins, insulin; Albi, albiglutide; Lira, liraglutide; Met, metformin; Tzd, thiazolidinedione; Pla, placebo; Su, sulfonylurea; DPP4i, DPP4 inhibitor; Sita, sitagliptin.
Figure 2
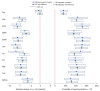
Summary of network meta-analysis results: absolute change in A1C and probability of reaching <7% treatment target. Notes: RE, random effect; CrI, credible interval; Pla, placebo; Albi, albiglutide; Dula, dulaglutide; EBID, exenatide bis in die (twice daily); EQW, exenatide quaque week (once weekly); Lira, liraglutide; Su, sulfonylurea; Ins, insulin; Met, metformin; Tzd, thiazolidinedione; DPP4i, dipeptidyl peptidase-4 inhibitor.
Figure 3
Direct meta-analysis results: odds of reaching <7% treatment target for exenatide 10 μg twice daily versus placebo. Abbreviations: OR, odds ratio; CI, confidence interval; DL, DerSimonian–Laird (random-effect model); MH, Mantel–Haenszel (fixed-effect model).
Similar articles
- Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis.
Htike ZZ, Zaccardi F, Papamargaritis D, Webb DR, Khunti K, Davies MJ. Htike ZZ, et al. Diabetes Obes Metab. 2017 Apr;19(4):524-536. doi: 10.1111/dom.12849. Epub 2017 Feb 17. Diabetes Obes Metab. 2017. PMID: 27981757 Review. - GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art.
Nauck MA, Quast DR, Wefers J, Meier JJ. Nauck MA, et al. Mol Metab. 2021 Apr;46:101102. doi: 10.1016/j.molmet.2020.101102. Epub 2020 Oct 14. Mol Metab. 2021. PMID: 33068776 Free PMC article. Review. - Treatment Patterns and Persistence With GLP-1 RA Treatments Among Patients With Type 2 Diabetes in France: A Retrospective Cohort Analysis.
Zimner Rapuch S, Divino V, Norrbacka K, Boye K, Lebrec J, Rosilio M, DeKoven M, Guerci B. Zimner Rapuch S, et al. Diabetes Ther. 2021 May;12(5):1553-1567. doi: 10.1007/s13300-021-01055-5. Epub 2021 Apr 17. Diabetes Ther. 2021. PMID: 33864629 Free PMC article. - A Network Meta-analysis Comparing Exenatide Once Weekly with Other GLP-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus.
Kayaniyil S, Lozano-Ortega G, Bennett HA, Johnsson K, Shaunik A, Grandy S, Kartman B. Kayaniyil S, et al. Diabetes Ther. 2016 Mar;7(1):27-43. doi: 10.1007/s13300-016-0155-1. Epub 2016 Feb 17. Diabetes Ther. 2016. PMID: 26886440 Free PMC article. Review.
Cited by
- Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis.
Yao H, Zhang A, Li D, Wu Y, Wang CZ, Wan JY, Yuan CS. Yao H, et al. BMJ. 2024 Jan 29;384:e076410. doi: 10.1136/bmj-2023-076410. BMJ. 2024. PMID: 38286487 Free PMC article. - Short-Term Effectiveness and Reduction in Insulin Requirements in Patients With Type 2 Diabetes Treated With IdegLira in a Real-World Setting.
Ramírez-Rincón A, Builes-Montaño CE, Hincapié-García JA, Blanco VM, Botero-Arango JF. Ramírez-Rincón A, et al. Front Endocrinol (Lausanne). 2022 Apr 28;13:828607. doi: 10.3389/fendo.2022.828607. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35573995 Free PMC article. - Design of Potent and Proteolytically Stable Biaryl-Stapled GLP-1R/GIPR Peptide Dual Agonists.
Yang Y, Lee C, Reddy RR, Huang DJ, Zhong W, Nguyen-Tran VTB, Shen W, Lin Q. Yang Y, et al. ACS Chem Biol. 2022 May 20;17(5):1249-1258. doi: 10.1021/acschembio.2c00175. Epub 2022 Apr 13. ACS Chem Biol. 2022. PMID: 35417146 Free PMC article. - A phase 1 multiple-ascending dose study of tirzepatide in Japanese participants with type 2 diabetes.
Furihata K, Mimura H, Urva S, Oura T, Ohwaki K, Imaoka T. Furihata K, et al. Diabetes Obes Metab. 2022 Feb;24(2):239-246. doi: 10.1111/dom.14572. Epub 2021 Nov 18. Diabetes Obes Metab. 2022. PMID: 34647404 Free PMC article. Clinical Trial. - Cardiovascular Risk Factor Status in Hospitalized Patients With Type 2 Diabetes in China.
Yang X, Liu Q, Fan Y, Ding L, Wang R, Hu G, Liu M. Yang X, et al. Front Endocrinol (Lausanne). 2021 Jul 22;12:664183. doi: 10.3389/fendo.2021.664183. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34367063 Free PMC article.
References
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015 – a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. - PubMed
- Canadian Diabetes Association Clinical Practice Guidelines Expert C, Imran SA, Rabasa-Lhoret R, Ross S. Targets for glycemic control. Can J Diabetes. 2013;37(Suppl 1):S31–S34. - PubMed
- International Diabetes Federation, Clinical Guidelines Task Force Global guideline for type 2 diabetes. [Accessed October 2015]. Available from http://www.idf.org/guideline-type-2-diabete.
- Internal Clinical Guidelines Team, National Institute for Health and Care Excellence Clinical guideline update. Type 2 diabetes in adults: management. [Accessed October 2015]. Available from http://www.nice.org.uk/guidance/indevel-opment/gid-cgwave0612/documents.
LinkOut - more resources
Full Text Sources
Other Literature Sources