A three-dimensional model of human lung development and disease from pluripotent stem cells - PubMed (original) (raw)
. 2017 May;19(5):542-549.
doi: 10.1038/ncb3510. Epub 2017 Apr 24.
Ya-Wen Chen 1 2 3, Sarah Xuelian Huang 1 2 3, Ana Luisa Rodrigues Toste de Carvalho 1 2 3 4 5, Mohammad Naimul Islam 3, Stefano Volpi 6 7, Luigi D Notarangelo 6, Michael Ciancanelli 8, Jean-Laurent Casanova 8, Jahar Bhattacharya 3 9, Alice F Liang 10, Laura M Palermo 11 12, Matteo Porotto 11 12, Anne Moscona 9 11 12 13, Hans-Willem Snoeck 1 2 3 13
Affiliations
- PMID: 28436965
- PMCID: PMC5777163
- DOI: 10.1038/ncb3510
A three-dimensional model of human lung development and disease from pluripotent stem cells
Ya-Wen Chen et al. Nat Cell Biol. 2017 May.
Abstract
Recapitulation of lung development from human pluripotent stem cells (hPSCs) in three dimensions (3D) would allow deeper insight into human development, as well as the development of innovative strategies for disease modelling, drug discovery and regenerative medicine. We report here the generation from hPSCs of lung bud organoids (LBOs) that contain mesoderm and pulmonary endoderm and develop into branching airway and early alveolar structures after xenotransplantation and in Matrigel 3D culture. Expression analysis and structural features indicated that the branching structures reached the second trimester of human gestation. Infection in vitro with respiratory syncytial virus, which causes small airway obstruction and bronchiolitis in infants, led to swelling, detachment and shedding of infected cells into the organoid lumens, similar to what has been observed in human lungs. Introduction of mutation in HPS1, which causes an early-onset form of intractable pulmonary fibrosis, led to accumulation of extracellular matrix and mesenchymal cells, suggesting the potential use of this model to recapitulate fibrotic lung disease in vitro. LBOs therefore recapitulate lung development and may provide a useful tool to model lung disease.
Conflict of interest statement
Competing Financial Interests
The authors have no competing financial interests.
Figures
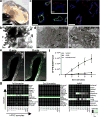
Figure 1. Generation of lung bud organoids
(a) Development of adherent structures during ventralization of AFE between d6 and d8 (see protocol Supplementary Fig. 1b), that could be expanded in suspension culture (d10, d20). Representative of >50 independent experiments (ESCs and iPSCs). Scale bars 250 µm. (b) Cellular expansion during the generation of LBOs (mean±s.e.m, n=3 independent experiments in RUES2 ESCs). The source data can be found in Supplementary Table 4. (c) Expression of EPCAM, KRT8, NKX2.1, FOXA1, and P63 in d25 LBOs. Representative of >10 independent experiments in ESCs and iPSCs. Scale bars 100 µm. (d) Staining of d25 LBO for ECADH and PDGFRA. Representative of 3 independent experiments in RUES2 ESCs. Scale bar 250 µm. (e) Expression of endodermal and mesodermal markers in the EPCAM+ and EPCAM− fraction of d25 LBOs determined by RNAseq (3 independent biological replicates, RUES2 ESCs).
Figure 2. In vivo potential of LBOs
(a) Macroscopic aspect of growths 1.5 months after transplantation of 106 LBO cells embedded in Matrigel under the kidney capsule of NSG mice. Scale bar 1 cm. (b) HE stain of LBO-derived growth 1.5 months after transplantation. Scale bar 500 µm. (c) Immunofluorescence for indicated markers in LBO-derived growths 1.5 months after transplantation. Scale bars 100 µm. (d) HE staining of LBO-derived growths 5 months after transplantation. Scale bars 250 µm. (e) Immunofluorescence for indicated markers in LBO-derived growth 5 months after transplantation. Scale bars 250 µm. (f) Dot blots for proteins marked on the left in aspirates from tubules in LBO-derived growth 5 months after transplantation. (g) HE staining and immunofluorescence for indicated markers in LBO-derived growths 7 months after transplantation. Scale bars 100 µm. All panels used RUES2 ESCs, representative of 4 independent experiments.
Figure 3. LBO differentiation in Matrigel at d70
(a) Bright field images of the development of an LBO into a branching structure after plating in Matrigel. RUES2 ESCs. Representative of >50 independent experiments. Scale bars 500 µm. (b) Immunofluorescence staining for indicated markers in d70 RUES2-derived LBOs plated in Matrigel at d25. Representative of 4 independent experiments. Scale bars 250 µm.
Figure 4. Long-term development of LBOs in vitro
(a) Macroscopic appearance of d170 RUES2 LBOs embedded in Matrigel at d25. Representative of >50 independent experiments. Scale bar 5 mm. (b) Bright field images of d170 RUES2 and C12 LBOs embedded in Matrigel at d25. Representative of >50 independent experiments. Scale bars 500 µm. (c) Immunofluorescence for indicated markers in d170 RUES2 LBOs embedded in Matrigel at d25. Representative of 3 independent experiments. Scale bars for MUC1+SFTPB and HT2-280 100 µm. Scale bar for SFTPC 10 µm. (d) Electron microscopy of d170 LBOs embedded in Matrigel at d25 in RUES2 ESCs and HDF SV iPSCs. Arrows indicate LBs. Representative of 3 independent experiments. (e) Uptake of SFTPB-BODIPY (green) in d170 LBOs embedded in Matrigel at d25. Representative of 4 independent experiments. Scale bars 100 µm. (f) Time-course of uptake of SFTPB-BODIPY in d170 LBOs embedded in Matrigel at d25 (mean±s.e.m, n=4 independent experiments in RUES2 ESCs). The source data can be found in Supplementary Table 4. (g) Comparison of genome-wide expression in d170 LBOs derived from hESCs and hiPSCs (12 biologically independent samples) with the KeyGenes database, showing the best match with second trimester human lung.
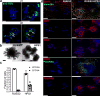
Figure 5. Potential application of LBOs in modeling human diseases
(a) Confocal images of whole mount d170 LBOs 1 and 2 days after infection with RSV and stained using anti-RSV (all antigens) antibody. Arrows: infected cells in the lumen. Representative of 3 independent experiments. Scale bars 100 µm. (b) Bright field images of d50 LBO-derived Matrigel colonies from RUES2 and RUES2-HPS1 cells. Representative of six independent experiments. Scale bars 500 µm. (c) Fraction of EPCAM+ and EPCAM− cells in d50 LBO-derived colonies in 3D Matrigel cultures of RUES2 and RUES2-HPS1 cells. (n=6, mean±s.e.m of 3 technical replicates from two experiments; * P<0.0001; two-tailed Student’s _t_-test). The source data can be found in Supplementary Table 4. (d) Immunofluorescence staining for mesenchymal markers and ECM components in 3D Matrigel cultures of RUES2 and RUES2-HPS1 cells. Representative of 3 independent experiments. Scale bars 500 µm.
Similar articles
- Authentic Modeling of Human Respiratory Virus Infection in Human Pluripotent Stem Cell-Derived Lung Organoids.
Porotto M, Ferren M, Chen YW, Siu Y, Makhsous N, Rima B, Briese T, Greninger AL, Snoeck HW, Moscona A. Porotto M, et al. mBio. 2019 May 7;10(3):e00723-19. doi: 10.1128/mBio.00723-19. mBio. 2019. PMID: 31064833 Free PMC article. - Generation of lung organoids from human pluripotent stem cells in vitro.
Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, Spence JR. Miller AJ, et al. Nat Protoc. 2019 Feb;14(2):518-540. doi: 10.1038/s41596-018-0104-8. Nat Protoc. 2019. PMID: 30664680 Free PMC article. - In vitro generation of human pluripotent stem cell derived lung organoids.
Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, White ES, Deutsch GH, Spence JR. Dye BR, et al. Elife. 2015 Mar 24;4:e05098. doi: 10.7554/eLife.05098. Elife. 2015. PMID: 25803487 Free PMC article. - Human pluripotent stem cell-derived lung organoids: Potential applications in development and disease modeling.
Tian L, Gao J, Garcia IM, Chen HJ, Castaldi A, Chen YW. Tian L, et al. Wiley Interdiscip Rev Dev Biol. 2021 Nov;10(6):e399. doi: 10.1002/wdev.399. Epub 2020 Nov 3. Wiley Interdiscip Rev Dev Biol. 2021. PMID: 33145915 Review. - Directed differentiation of ureteric bud and collecting duct organoids from human pluripotent stem cells.
Shi M, Fu P, Bonventre JV, McCracken KW. Shi M, et al. Nat Protoc. 2023 Aug;18(8):2485-2508. doi: 10.1038/s41596-023-00847-2. Epub 2023 Jul 17. Nat Protoc. 2023. PMID: 37460630 Free PMC article. Review.
Cited by
- Human Lung Organoid Culture in Alginate With and Without Matrigel to Model Development and Disease.
Dye BR, Decker JT, Hein RFC, Miller AJ, Huang S, Spence JR, Shea LD. Dye BR, et al. Tissue Eng Part A. 2022 Nov;28(21-22):893-906. doi: 10.1089/ten.TEA.2022.0054. Epub 2022 Oct 6. Tissue Eng Part A. 2022. PMID: 36029210 Free PMC article. - Bioengineering tools to speed up the discovery and preclinical testing of vaccines for SARS-CoV-2 and therapeutic agents for COVID-19.
Raimondi MT, Donnaloja F, Barzaghini B, Bocconi A, Conci C, Parodi V, Jacchetti E, Carelli S. Raimondi MT, et al. Theranostics. 2020 May 27;10(16):7034-7052. doi: 10.7150/thno.47406. eCollection 2020. Theranostics. 2020. PMID: 32641977 Free PMC article. Review. - From Clones to Buds and Branches: The Use of Lung Organoids to Model Branching Morphogenesis Ex Vivo.
Vazquez-Armendariz AI, Herold S. Vazquez-Armendariz AI, et al. Front Cell Dev Biol. 2021 Mar 4;9:631579. doi: 10.3389/fcell.2021.631579. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748115 Free PMC article. Review. - Influence of mesenchymal and biophysical components on distal lung organoid differentiation.
Goltsis O, Bilodeau C, Wang J, Luo D, Asgari M, Bozec L, Pettersson A, Leibel SL, Post M. Goltsis O, et al. Stem Cell Res Ther. 2024 Sep 2;15(1):273. doi: 10.1186/s13287-024-03890-2. Stem Cell Res Ther. 2024. PMID: 39218985 Free PMC article. - Reconstructing the pulmonary niche with stem cells: a lung story.
Varghese B, Ling Z, Ren X. Varghese B, et al. Stem Cell Res Ther. 2022 Apr 11;13(1):161. doi: 10.1186/s13287-022-02830-2. Stem Cell Res Ther. 2022. PMID: 35410254 Free PMC article. Review.
References
- Mulugeta S, Nureki S, Beers MF. Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease. American journal of physiology. Lung cellular and molecular physiology. 2015;309:L507–525. doi: 10.1152/ajplung.00139.2015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 RR027050/RR/NCRR NIH HHS/United States
- R01 AI076335/AI/NIAID NIH HHS/United States
- R01 AI114736/AI/NIAID NIH HHS/United States
- S10 OD020056/OD/NIH HHS/United States
- U01 HL134760/HL/NHLBI NIH HHS/United States
- R01 HL120046/HL/NHLBI NIH HHS/United States
- R01 AI031971/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical