Voltage imaging with genetically encoded indicators - PubMed (original) (raw)
Review
Voltage imaging with genetically encoded indicators
Yongxian Xu et al. Curr Opin Chem Biol. 2017 Aug.
Abstract
Membrane voltages are ubiquitous throughout cell biology. Voltage is most commonly associated with excitable cells such as neurons and cardiomyocytes, although many other cell types and organelles also support electrical signaling. Voltage imaging in vivo would offer unique capabilities in reporting the spatial pattern and temporal dynamics of electrical signaling at the cellular and circuit levels. Voltage is not directly visible, and so a longstanding challenge has been to develop genetically encoded fluorescent voltage indicator proteins. Recent advances have led to a profusion of new voltage indicators, based on different scaffolds and with different tradeoffs between voltage sensitivity, speed, brightness, and spectrum. In this review, we describe recent advances in design and applications of genetically-encoded voltage indicators (GEVIs). We also highlight the protein engineering strategies employed to improve the dynamic range and kinetics of GEVIs and opportunities for future advances.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
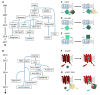
Figure 1. The family of GEVIs
A) and B) Approximate lineages of the major classes of GEVIs. The GEVIs highlighted in bold have shown the greatest sensitivity for use in vivo and are colored with their approximate excitation wavelengths. A) GEVIs based on voltage-sensor domains. B) GEVIs based on microbial rhodopsins. C)–G) Voltage sensing mechanisms in the major classes of GEVIs.
Figure 2. Applications of GEVI imaging
A) All-optical electrophysiology (‘Optopatch’) in neurons co-expressing a blue-shifted channelrhodopsin, CheRiff, and a red-shifted NIR GEVI, QuasAr2. Left: Optical stimuli evoke electrical spikes and closely corresponding fluorescence transients. Right: Patterned optogenetic stimulation on a dendritic region evokes action potentials whose sub-cellular propagation initiates at the axon initial segment, marked by an Ankyrin G immunostain. Figures from Ref. [32]. B) Ace2N-mNeon reports neuronal spikes in mouse visual cortex in vivo. Figure from Ref. [36]. C) Two-photon imaging of ASAP2f in Drosophila visual neurons reports sub-cellular details of stimulus-evoked electrical responses. Figure from Ref. [20]. D) Wide-field optical voltage mapping in mouse cortex in vivo. The optical signal correlates with the EEG from the ipsilateral but not the contralateral hemisphere. Figure from Ref. [16].
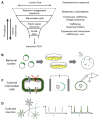
Figure 3. GEVI screening pipeline
A) A hierarchical approach screens large libraries for the most easily measured parameters (brightness), and then characterizes hits for voltage sensitivity and speed. Trafficking must ultimately be tested by GEVI expression in vivo and imaging in acute slice. Figure modified from Ref. [56]. B) Bacterial screens for brightness can be performed in a pooled library assay where highly fluorescent colonies are manually selected for propagation. To distinguish brightness from colony size, it is important to have a spectrally distinct reference fluorophore expressed at a constant level. C) Tests for voltage sensitivity and kinetics can be performed in cultured mammalian cells, using either (left) spiking HEK cells, (middle) induced transmembrane voltage, or (right) manual patch-clamp electrophysiology. D) Tests in cultured neurons probe trafficking and high-speed kinetics. Neural activity can be induced either optogenetically or via field-stimulation electrodes.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Dynamic Field Theory of Executive Function: Identifying Early Neurocognitive Markers.
McCraw A, Sullivan J, Lowery K, Eddings R, Heim HR, Buss AT. McCraw A, et al. Monogr Soc Res Child Dev. 2024 Dec;89(3):7-109. doi: 10.1111/mono.12478. Monogr Soc Res Child Dev. 2024. PMID: 39628288 Free PMC article. - Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.
Elwenspoek MM, Thom H, Sheppard AL, Keeney E, O'Donnell R, Jackson J, Roadevin C, Dawson S, Lane D, Stubbs J, Everitt H, Watson JC, Hay AD, Gillett P, Robins G, Jones HE, Mallett S, Whiting PF. Elwenspoek MM, et al. Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article. - Macrolide antibiotics (including azithromycin) for cystic fibrosis.
Southern KW, Solis-Moya A, Kurz D, Smith S. Southern KW, et al. Cochrane Database Syst Rev. 2024 Feb 27;2(2):CD002203. doi: 10.1002/14651858.CD002203.pub5. Cochrane Database Syst Rev. 2024. PMID: 38411248 Review. - Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.
Manes TJ, DeGenova DT, Taylor BC, Patel JN. Manes TJ, et al. JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
Cited by
- Imaging Spontaneous Neuronal Activity with Voltage-Sensitive Dyes.
Raliski BK, Kirk MJ, Miller EW. Raliski BK, et al. Curr Protoc. 2021 Mar;1(3):e48. doi: 10.1002/cpz1.48. Curr Protoc. 2021. PMID: 33760396 Free PMC article. - Dual-Color Optical Recording of Bioelectric Potentials by Polymer Electrochromism.
Zhou Y, Liu E, Yang Y, Alfonso FS, Ahmed B, Nakasone K, Forró C, Müller H, Cui B. Zhou Y, et al. J Am Chem Soc. 2022 Dec 28;144(51):23505-23515. doi: 10.1021/jacs.2c10198. Epub 2022 Dec 16. J Am Chem Soc. 2022. PMID: 36525312 Free PMC article. - Cognitive neuroscience perspective on memory: overview and summary.
Sridhar S, Khamaj A, Asthana MK. Sridhar S, et al. Front Hum Neurosci. 2023 Jul 26;17:1217093. doi: 10.3389/fnhum.2023.1217093. eCollection 2023. Front Hum Neurosci. 2023. PMID: 37565054 Free PMC article. - High-Accuracy Detection of Neuronal Ensemble Activity in Two-Photon Functional Microscopy Using Smart Line Scanning.
Brondi M, Moroni M, Vecchia D, Molano-Mazón M, Panzeri S, Fellin T. Brondi M, et al. Cell Rep. 2020 Feb 25;30(8):2567-2580.e6. doi: 10.1016/j.celrep.2020.01.105. Cell Rep. 2020. PMID: 32101736 Free PMC article. - QuasAr Odyssey: the origin of fluorescence and its voltage sensitivity in microbial rhodopsins.
Silapetere A, Hwang S, Hontani Y, Fernandez Lahore RG, Balke J, Escobar FV, Tros M, Konold PE, Matis R, Croce R, Walla PJ, Hildebrandt P, Alexiev U, Kennis JTM, Sun H, Utesch T, Hegemann P. Silapetere A, et al. Nat Commun. 2022 Sep 20;13(1):5501. doi: 10.1038/s41467-022-33084-4. Nat Commun. 2022. PMID: 36127376 Free PMC article.
References
- Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. - PubMed
- Cohen AE, Venkatachalam V. Bringing bioelectricity to light. Annu Rev Biophys. 2014;43:211–232. - PubMed
- Kralj JM, Hochbaum DR, Douglass AD, Cohen AE. Electrical spiking in Escherichia coli probed with a fluorescent voltage-indicating protein. Science. 2011;333:345–348. The discovery of microbial rhodopsins as a novel GEVI scaffold. This probe revealed surprising spontaneous voltage fluctuations in bacteria. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources