Type Iγ phosphatidylinositol phosphate kinase regulates PD-L1 expression by activating NF-κB - PubMed (original) (raw)
Type Iγ phosphatidylinositol phosphate kinase regulates PD-L1 expression by activating NF-κB
Junli Xue et al. Oncotarget. 2017.
Abstract
The programmed death-ligand 1 (PD-L1), by binding to PD-1 on the surface of immune cells, activates a major immune checkpoint pathway. Elevated expression of PD-L1 in tumor cells mediates tumor-induced T-cell exhaustion and immune suppression; therefore protect the survival of tumor cells. Although blockade of the PD-1/PD-L1 axis exhibits great potential in cancer treatment, mechanisms driving the up-regulation of PD-L1 in tumor cells remain not fully understood. Here we found that type Iγ phosphatidylinositol 4-phosphate (PtdIns(4)P) 5-kinase (PIPKIγ) is required for PD-L1 expression in triple negative breast cancer cells. Depletion of PIPKIγ inhibits both intrinsic and induced PD-L1 expression. Results from further analyses suggest that PIPKIγ promotes the transcription of the PD-L1 gene by activating the NF-κB pathway in these cells. These results demonstrate that PIPKIγ-dependent expression of PD-L1 is likely important for the progression of triple negative breast cancer.
Keywords: AKT; NF-κB; PD-L1; PIPKIγ; triple negative breast cancer.
Conflict of interest statement
CONFLICTS OF INTEREST
None.
Figures
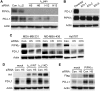
Figure 1. PIPKIγ-depleted TNBC cells exhibits downregulated PD-L1 expression
(A) MDA-MB-231 cells were transfected with non-specific control (Con.), human pan-PIPKIγ (Iγ-pan, 4 distinct siRNAs including H5, H6, H12, H13), or human PIPKIγ_i2-specific (Iγ_i2) siRNAs, respectively. 48 hrs post transfections, cells were collected for immunoblotting analyses using indicated antibodies. (B) Three PD-L1-null MDA-MB-231 single clones (KO-1, KO-2, KO-6) were constructed using CRISPR/Cas9 system. PIPKIγ expression in the parental and individual PD-L1-null cell lines was determined by immunoblotting. (C) Three different human TNBC cell lines were transfected with control or pan-PIPKIγ siRNAs (H5 and H13) and then PD-L1 expression was accessed by immunoblotting. (D) Hs578T cells were transfected to express control empty vector (mock), H5-resistant mouse PIPKIγ_i1 wild type (Iγ_i1WT) or kinase dead (Iγ_i1KD) for 24 hrs, and then transfected with control (Con.) or HA-tagged human pan-PIPKIγ specific (H5) siRNAs for another 48 hrs. These cells were then lysed and subjected to immunoblotting with indicated antibodies. (E) Hs578T cells were handled as described in (D) except for using Flag-tagged human PIPKIα to replace HA-tagged mouse PIPKIγ_i1 construct.
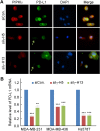
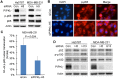
Figure 2. Loss of PIPKIγ inhibits PD-L1 transcription
(A) After transfected with control (Ctrl) or pan-PIPKIγ-specific siRNAs (H5 and H13) for 48 hrs, MDA-MB-231 cells were subjected to indirect immunofluorescence with antibodies against PD-L1 (green), PIPKIγ (Iγ855, red), and DAPI (blue), and then analyzed under epi-fluorescence microscope. Arrows indicate the PIPKIγ–depleted cells where PD-L1 showed substantially decreased signal comparing to control cells expressing normal level of PIPKIγ. (B) Three different TNBC cell lines were transfected with control or pan-PIPKIγ siRNAs (H5 and H13) for 48 hrs. Then the levels of PD-L1 mRNA in cells of each group were accessed by quantitative RT-PCR (qRT-PCR) using specific PD-L1 primers with GAPDH as the internal control. Results from at least three independent experiments were quantified, statistically analyzed, and plotted as mean ± S.E.M.
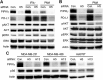
Figure 3. PIPKIγ regulates the induced-expression of PD-L1 in TNBC cells independent of AKT
(A) 48 hrs after transfection with control (Ctrl) or pan-PIPKIγ (H5) siRNA, Hs578T cells were treated with 10 ng/mL PMA for 12 hrs or 30 ng/mL IFN-γ for 48 hrs. Then cells were lysed and analyzed by immunoblotting using indicated antibodies. Iγ855, pan-PIPKIγ; pAKT, phospho-AKT (pAKT); pERK (phospho-ERK). (B) MDA-MB-231 cells were transfected with control or pan-PIPKIγ (H5) siRNA for 48 hrs and then stimulated with 10 ng/mL PMA for 12 hrs. Cells were then subjected to immunoblotting analyses as described in A. (C) Three TNBC cell lines were treated with control or pan-PIPKIγ (H5 and H13) siRNAs for 48 hrs and then analyzed by immunoblotting with indicated antibodies. pS6, phosphorylated S6.
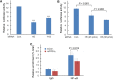
Figure 4. PIPKIγ is required for NF-κB-mediated transcription in TNBC cells
(A) MDA-MB-231 cells were treated with control or pan-PIPKIγ (H5 or H13) siRNAs for 48 hrs, transfected with control vector pGL3-Basic or NF-κB reporter p1242-3x-κB-L together with pRL-SV40P for 24 hrs, and then subjected to luciferase assay. (B) MDA-MB-231 cells were treated with control or indicated amount of pan-PIPKIγ (H5) siRNAs for 48 hrs. These cells were then transfected and analyzed for dual-luciferase assay as described in A. (C) Control or PIPKIγ–depleted MDA-MB-231 cells were subjected to ChIP assay using anti-p65 antibody followed by PCR with PD-L1 specific primers. A–C, Results obtained from at least three independent experiments were quantified, statistically analyzed, and then plotted as mean ± s.d.
Figure 5. Depletion of PIPKIγ restrains the phosphorylation of p65
(A) MDA-MB-231 or Hs578T cells were treated with control (Ctrl) or pan-PIPKIγ (H5) siRNA for 48 hrs, and then analyzed by immunoblotting with indicated antibodies. p-p65, phosphorylated p65. (B) MDA-MB-231 cells as described in A were used for indirect immunofluorescence microscopy to determine the nuclear signal of phosphorylated p65. (C) Fluorescence intensity of p-p65 in the nucleus were quantified in > 100 cells of each group as described in B, then the results from three independent experiments were statistically analyzed and plotted as mean ± s.d. (D) MDA-MB-231 or Hs578T cells were transfected with control (Con.) or two different human pan-PIPKIγ-specific siRNAs (H5 and H13) for 48 hrs. Cells were then lysed and analyzed by Western blot using indicated antibodies. p-p100, Phosphorylated p100.
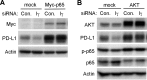
Figure 6. Overexpression of p65 or AKT recovered the downregulation of intrinsic PD-L1 expression caused by PIPKIγ depletion
Hs578T cells were transfected with Myc-tagged p65 (A) or AKT equally mixed with AKT1, AKT2 and AKT3 constructs (B) for 24 hrs, then treated with control (Con.) or human pan-PIPKIγ specific (H5) siRNAs for 48 hrs. Cells were then analyzed by Western blot with indicated antibodies. p-p65, phosphorylated p65.
Similar articles
- Syntenin1/MDA-9 (SDCBP) induces immune evasion in triple-negative breast cancer by upregulating PD-L1.
Liu J, Yang Y, Wang H, Wang B, Zhao K, Jiang W, Bai W, Liu J, Yin J. Liu J, et al. Breast Cancer Res Treat. 2018 Sep;171(2):345-357. doi: 10.1007/s10549-018-4833-8. Epub 2018 May 29. Breast Cancer Res Treat. 2018. PMID: 29845474 - Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells.
Wang X, Yang L, Huang F, Zhang Q, Liu S, Ma L, You Z. Wang X, et al. Immunol Lett. 2017 Apr;184:7-14. doi: 10.1016/j.imlet.2017.02.006. Epub 2017 Feb 14. Immunol Lett. 2017. PMID: 28223102 Free PMC article. - LPS promotes the expression of PD-L1 in gastric cancer cells through NF-κB activation.
Li H, Xia JQ, Zhu FS, Xi ZH, Pan CY, Gu LM, Tian YZ. Li H, et al. J Cell Biochem. 2018 Dec;119(12):9997-10004. doi: 10.1002/jcb.27329. Epub 2018 Aug 26. J Cell Biochem. 2018. PMID: 30145830 - Dual inhibition of STAT1 and STAT3 activation downregulates expression of PD-L1 in human breast cancer cells.
Sasidharan Nair V, Toor SM, Ali BR, Elkord E. Sasidharan Nair V, et al. Expert Opin Ther Targets. 2018 Jun;22(6):547-557. doi: 10.1080/14728222.2018.1471137. Epub 2018 May 2. Expert Opin Ther Targets. 2018. PMID: 29702007 Review. - Regulation of PD-L1 Expression by NF-κB in Cancer.
Antonangeli F, Natalini A, Garassino MC, Sica A, Santoni A, Di Rosa F. Antonangeli F, et al. Front Immunol. 2020 Nov 25;11:584626. doi: 10.3389/fimmu.2020.584626. eCollection 2020. Front Immunol. 2020. PMID: 33324403 Free PMC article. Review.
Cited by
- PIPKI_γ_ Regulates CCL2 Expression in Colorectal Cancer by Activating AKT-STAT3 Signaling.
Xue J, Ge X, Zhao W, Xue L, Dai C, Lin F, Peng W. Xue J, et al. J Immunol Res. 2019 Nov 3;2019:3690561. doi: 10.1155/2019/3690561. eCollection 2019. J Immunol Res. 2019. PMID: 31781676 Free PMC article. - PD-L1 Expression On tumor Cells Was Associated With Unfavorable Prognosis In Esophageal Squamous Cell Carcinoma.
Wang Q, Feng F, Wang F, Liu Z, Liu S, Xu G, Zheng G, Guo M, Lian X, Zhang H. Wang Q, et al. J Cancer. 2018 Jun 5;9(12):2224-2231. doi: 10.7150/jca.24493. eCollection 2018. J Cancer. 2018. PMID: 29937943 Free PMC article. - Targeting type Iγ phosphatidylinositol phosphate kinase overcomes oxaliplatin resistance in colorectal cancer.
Yu M, Wang H, Zhao W, Ge X, Huang W, Lin F, Tang W, Li A, Liu S, Li RK, Jiang SH, Xue J. Yu M, et al. Theranostics. 2022 May 20;12(9):4386-4398. doi: 10.7150/thno.69863. eCollection 2022. Theranostics. 2022. PMID: 35673560 Free PMC article. - Roles of CCL2-CCR2 Axis in the Tumor Microenvironment.
Kadomoto S, Izumi K, Mizokami A. Kadomoto S, et al. Int J Mol Sci. 2021 Aug 8;22(16):8530. doi: 10.3390/ijms22168530. Int J Mol Sci. 2021. PMID: 34445235 Free PMC article. Review. - Immune checkpoint molecules: "new" kids on the block of skin photoimmunology.
Wang W, Wu ZH. Wang W, et al. Genes Dis. 2019 Dec 4;8(1):1-5. doi: 10.1016/j.gendis.2019.11.002. eCollection 2021 Jan. Genes Dis. 2019. PMID: 33569508 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials