Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota - PubMed (original) (raw)
Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota
Da Zhou et al. Sci Rep. 2017.
Abstract
Non-alcoholic steatohepatitis (NASH) is an epidemic metabolic disease with limited therapeutic strategies. Cumulative data support the pivotal role of gut microbiota in NASH. Here, we investigated the hypothesis regarding whether fecal microbiota transplantation (FMT) is effective in attenuating high-fat diet (HFD)-induced steatohepatitis in mice. Mice were randomized into control, HFD and HFD + FMT groups. After an 8-week HFD, FMT treatment was initiated and carried out for 8 weeks. The gut microbiota structure, butyrate concentrations of the cecal content, liver pathology and intrahepatic lipid and cytokines were examined. Our results showed that after FMT, the gut microbiota disturbance was corrected in HFD-fed mice with elevated abundances of the beneficial bacteria Christensenellaceae and Lactobacillus. FMT also increased butyrate concentrations of the cecal content and the intestinal tight junction protein ZO-1, resulting in relief of endotoxima in HFD-fed mice. Steatohepatitis was alleviated after FMT, as indicated by a significant decrease in intrahepatic lipid accumulation (reduced Oli-red staining, decreased intrahepatic triglyceride and cholesterol), intrahepatic pro-inflammatory cytokines, and the NAS score. Accordingly, intrahepatic IFN-γ and IL-17 were decreased, but Foxp3, IL-4 and IL-22 were increased after FMT intervention. These data indicate that FMT attenuated HFD-induced steatohepatitis in mice via a beneficial effect on the gut microbiota.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
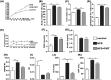
FMT attenuated HFD-induced obesity, liver injury and metabolic disturbance. (A,B) Body weight changes and body weight at 16th week. (C) Liver index = liver weight/body weight × 100. (D) Epididymal fat index = epididymal fat weight/body weight × 100. (E) Energy intake per mouse per week. (F,I) Fasting serum glucose, fasting serum insulin, HOMA-IR and ISI of the three groups. J,K) Serum ALT and AST. The data represent the mean ± S.E.M. (n = 12 mice per group), * P < 0.05, ** P < 0.01 and *** P < 0.001.
Figure 2
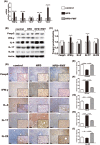
Influences of FMT on gut microbiota. (A,B) Scatter plot of the unweighted-unifrac-principal co-ordinates analysis (PCOA) score showing the similarity of the 18 bacterial communities based on the Unifrac distance and Scatter plot of the weighted-PCOA score. (C) Nonmetric multidimensional scaling (NMDS) showing the difference in bacterial communities according to the Bray-Curtis distance. (D) Hierarchical cluster analysis. (E,F) Bacterial composition of the different communities at the genus level (E) and at phylum level (F). Sequences that could not be classified into any known group were assigned as “no rank.” (G,H) Differences were represented in the color of the most abundant group. Key phylotypes of the gut microbiota responding to FMT treatment (G), the histogram (H) showing the lineages with LDA values as determined by LEfSe. (I,K) The acetic acid, propanoic acid and butyrate acid levels of the cecal content. The data represent the mean ± S.E.M. (n = 12 mice per group), *** P < 0.001.
Figure 3
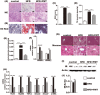
Beneficial effects of FMT on the small intestine and the level of serum endotoxin. (A) HE staining of the small intestine showing that FMT restored HFD-induced mucosal damage. (B) Immunohistochemistry for ZO-1 showing that FMT increased ZO-1 expression. (C) ZO-1 mRNA expression in the small intestine. (D) The level of serum endotoxin. The data represent the mean ± S.E.M. (n = 12 mice per group), * P < 0.05, ** P < 0.01 and *** P < 0.001.
Figure 4
FMT improved inflammation and lipid metabolism in liver. (A,B) HE and oil red O staining of liver. (C) NAS score. (D,E) the level of TG and cholesterol in the liver. (F) Lipid metabolism-associated PPAR-α and PPAR-γ gene expression in the liver. (G) Masson’s staining. (H) Endotoxin-associated gene expression of TLR4 and Myd88 and fibrosis-associated gene expression of TGF-β, Smad2, Smad7 and α-SMA. (I,J) IR protein expression level in liver. Gene expression levels are expressed as values relative to the control group. The data represent the mean ± S.E.M. (n = 12 mice per group), * P < 0.05, ** P < 0.01 and *** P < 0.001.
Figure 5
Immunoregulation of FMT in the liver. (A) Pro-inflammation-associated gene expression in the liver. (B,C) Foxp3, IFN-γ, IL-4, IL-17 and IL-22 protein expression levels in the liver. (D–I) Immunohistochemistry for Foxp3, IFN-γ, IL-4, IL-17 and IL-22 in the liver showed that Foxp3, IL-4, and IL-22 were increased and IFN-γ and IL-17 were decreased after FMT intervention. The data represent the mean ± S.E.M. * P < 0.05, ** P < 0.01 and *** P < 0.001.
Figure 6
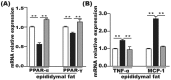
FMT improved inflammation and lipid metabolism in epididymal fat tissue. (A) Lipid metabolism-associated PPAR-α and PPAR-γ gene expression in the epididymal fat tissue. (B) Gene expression levels of MCP-1 and TNF-α in the epididymal fat tissue. Gene expression levels are expressed as values relative to the control group. The data represent the mean ± S.E.M. (n = 12 mice per group), * P < 0.05, ** P < 0.01 and *** P < 0.001.
Similar articles
- Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier.
Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, Liu C, Chen YW, Fan JG. Zhou D, et al. World J Gastroenterol. 2017 Jan 7;23(1):60-75. doi: 10.3748/wjg.v23.i1.60. World J Gastroenterol. 2017. PMID: 28104981 Free PMC article. - Lingguizhugan decoction attenuates diet-induced obesity and hepatosteatosis via gut microbiota.
Liu MT, Huang YJ, Zhang TY, Tan LB, Lu XF, Qin J. Liu MT, et al. World J Gastroenterol. 2019 Jul 21;25(27):3590-3606. doi: 10.3748/wjg.v25.i27.3590. World J Gastroenterol. 2019. PMID: 31367159 Free PMC article. - Hepatobiliary and pancreatic: Multi-donor fecal microbiota transplantation attenuated high-fat diet-induced hepatic steatosis in mice by remodeling the gut microbiota.
Shou D, Luo Q, Tang W, Cao C, Huang H, Chen H, Zhou Y. Shou D, et al. J Gastroenterol Hepatol. 2023 Dec;38(12):2195-2205. doi: 10.1111/jgh.16359. Epub 2023 Oct 3. J Gastroenterol Hepatol. 2023. PMID: 37787118 - Mechanistic and physiological approaches of fecal microbiota transplantation in the management of NAFLD.
Gupta M, Krishan P, Kaur A, Arora S, Trehanpati N, Singh TG, Bedi O. Gupta M, et al. Inflamm Res. 2021 Jul;70(7):765-776. doi: 10.1007/s00011-021-01480-z. Epub 2021 Jul 1. Inflamm Res. 2021. PMID: 34212214 Review. - Fecal microbiota transplantation: a promising strategy in preventing the progression of non-alcoholic steatohepatitis and improving the anti-cancer immune response.
Delaune V, Orci LA, Lacotte S, Peloso A, Schrenzel J, Lazarevic V, Toso C. Delaune V, et al. Expert Opin Biol Ther. 2018 Oct;18(10):1061-1071. doi: 10.1080/14712598.2018.1518424. Epub 2018 Sep 10. Expert Opin Biol Ther. 2018. PMID: 30173562 Review.
Cited by
- Diagnosing and engineering gut microbiomes.
Cappio Barazzone E, Diard M, Hug I, Larsson L, Slack E. Cappio Barazzone E, et al. EMBO Mol Med. 2024 Nov;16(11):2660-2677. doi: 10.1038/s44321-024-00149-4. Epub 2024 Oct 28. EMBO Mol Med. 2024. PMID: 39468301 Free PMC article. Review. - A Scoping Review on Hepatoprotective Mechanism of Herbal Preparations through Gut Microbiota Modulation.
Poo CL, Lau MS, Nasir NLM, Nik Zainuddin NAS, Rahman MRAA, Mustapha Kamal SK, Awang N, Muhammad H. Poo CL, et al. Curr Issues Mol Biol. 2024 Oct 16;46(10):11460-11502. doi: 10.3390/cimb46100682. Curr Issues Mol Biol. 2024. PMID: 39451562 Free PMC article. Review. - The Role of Gut Microbiota Modification in Nonalcoholic Fatty Liver Disease Treatment Strategies.
Yaghmaei H, Bahanesteh A, Soltanipur M, Takaloo S, Rezaei M, Siadat SD. Yaghmaei H, et al. Int J Hepatol. 2024 Oct 16;2024:4183880. doi: 10.1155/2024/4183880. eCollection 2024. Int J Hepatol. 2024. PMID: 39444759 Free PMC article. Review. - Gut microbiome therapy: fecal microbiota transplantation vs live biotherapeutic products.
Kim DY, Lee SY, Lee JY, Whon TW, Lee JY, Jeon CO, Bae JW. Kim DY, et al. Gut Microbes. 2024 Jan-Dec;16(1):2412376. doi: 10.1080/19490976.2024.2412376. Epub 2024 Oct 8. Gut Microbes. 2024. PMID: 39377231 Free PMC article. Review. - The role of probiotics on the roadmap to a healthy microbiota: a symposium report.
Lockyer S, Aguirre M, Durrant L, Pot B, Suzuki K. Lockyer S, et al. Gut Microbiome (Camb). 2020 Aug 26;1:e2. doi: 10.1017/gmb.2020.2. eCollection 2020. Gut Microbiome (Camb). 2020. PMID: 39296722 Free PMC article. Review.
References
- Rinella ME. Nonalcoholic Fatty Liver Disease. Jama. 2015;313(2263):5370–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical