Differential cardiovascular profiles of sodium-glucose cotransporter 2 inhibitors: critical evaluation of empagliflozin - PubMed (original) (raw)
Review
Differential cardiovascular profiles of sodium-glucose cotransporter 2 inhibitors: critical evaluation of empagliflozin
Vani P Sanon et al. Ther Clin Risk Manag. 2017.
Abstract
One of the most feared repercussions of type 2 diabetes mellitus is the risk of adverse cardiovascular outcomes. The current antidiabetic agents on the market have had difficulty in showing cardiovascular outcome improvement. The EMPA-REG OUTCOME trial studied the sodium-glucose cotransporter 2 inhibitor empagliflozin in type 2 diabetic patients at high risk of cardiovascular events. The trial results revealed a decrease in the composite primary end points of death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke in those taking empagliflozin vs placebo. Those taking the medication also had a significant decrease in death from any cause, death from cardiovascular cause, and hospitalization for heart failure. The EMPA-REG trial is paradigm shifting because it demonstrates a clear mortality benefit to cardiovascular outcomes with a low side-effect profile, in contrast to prior outcome studies of hypoglycemic agents. Further studies are required to better clarify the long-term safety and efficacy of this promising class of diabetic drugs.
Keywords: SGLT2 inhibitors; cardiovascular mortality; diabetes; heart failure; hypertension.
Conflict of interest statement
Disclosure Dr Robert Chilton serves on advisory boards and research for BI, Lilly, Takada, Pfizer, and MSD. The authors report no further conflicts of interest in this work.
Figures
Figure 1
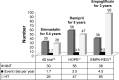
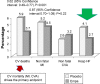
The recent hypoglycemia trials for diabetes with cardiovascular event rates per year. Note: Note the marked reduction in CV death, hospitalizations for heart failure, and all-cause mortality with empagliflozin. Abbreviations: CV, cardiovascular; SGLT2, sodium-glucose cotransporter 2; NS, nonsignificant; SAVOR, saxagliptin reduce the risk of cardiovascular events; EXAMINE, examination of cardiovascular outcomes with alogliptin versus standard of care; TECOS, trial evaluating cardiovascular outcomes with sitagliptin; EMPA-REG, empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes.
Figure 2
The macula densa and SGLT2 inhibitors. Notes: The effect of marked diuresis and flow changes could have major impact on activation of macular densa cells. Recent reports have noted significant changes in aldosterone levels. Clinical importance remains to be determined. Abbreviation: SGLT2, sodium-glucose cotransporter 2.
Figure 3
Three large CV outcome trials have increasing cardiovascular event rates as diabetes is added to high-risk patients. Notes: There is increase in the percentage of patients with hypertension in the more recent diabetes trials. 4S trial simvastatin: 182/2,221 (8.2%), placebo: 256/2,223 (11.5%) HR =0.71 (0.59–0.85), HOPE trial ramipril: 482/4,645 (10.4%), placebo: 569/4,652 (12.2%) HR =0.85 (0.76–0.95), EMPA-REG empa: 269 (5.7%)/2,333, placebo: 194 (8.3%)/4,687 HR =0.68 (0.57–0.82). The number needed to treat calculations find that empagliflozin in only 3 years has a NNT that is very close to 4S that needed 5.4 years to have NNT of 30. Abbreviations: CV, cardiovascular; HR, hazard ratio; NNT, number needed to treat; HT, hypertension.
Figure 4
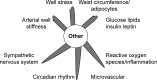
Potential mechanism reducing cardiovascular events.
Figure 5
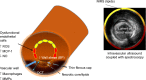
The vascular wall in patients with diabetes is well known to have advanced atherosclerosis. Notes: The wall stress increases stress on the diseased diabetes vascular wall leading to plaque fracture. The thin cap is not as elastic, and the stiffness of this plaque cap increases the risk for plaque rupture. In addition, the cap frequently is much thinner with a necrotic core as seen in the PROSPECT trial., Abbreviations: BP, blood pressure; NIRS, near infrared spectroscopy; ROS, reactive oxygen species; MCP-1, monocyte chemoattractant protein-1; MMP, matrix metalloproteinase; OCT, optical coherence tomography; NO, nitric oxide.
Figure 6
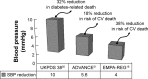
Reductions in systolic blood pressure are well known to reduce CV risk from multiple studies. Notes: These three large trials found similar benefits with the caveat that EMPA-REG has an impressive reduction in CV death. This suggests that SGLT2 inhibitors may have multiple mechanisms related to reducing CV events in diabetic patients. Abbreviations: CV, cardiovascular; SGLT2, sodium-glucose cotransporter 2; SBP, systolic blood pressure.
Figure 7
The significant reduction in both CV deaths and hospitalization for heart failure. Abbreviations: CV, cardiovascular; MI, myocardial infarction; CVA, cardiovascular accident; hosp, hospitalization; HF, heart failure.
Similar articles
- Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications.
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Heerspink HJ, et al. Circulation. 2016 Sep 6;134(10):752-72. doi: 10.1161/CIRCULATIONAHA.116.021887. Epub 2016 Jul 28. Circulation. 2016. PMID: 27470878 Review. - EMPA-REG and Other Cardiovascular Outcome Trials of Glucose-lowering Agents: Implications for Future Treatment Strategies in Type 2 Diabetes Mellitus.
Schernthaner G, Schernthaner-Reiter MH, Schernthaner GH. Schernthaner G, et al. Clin Ther. 2016 Jun;38(6):1288-1298. doi: 10.1016/j.clinthera.2016.04.037. Epub 2016 May 19. Clin Ther. 2016. PMID: 27210264 - Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Zinman B, et al. N Engl J Med. 2015 Nov 26;373(22):2117-28. doi: 10.1056/NEJMoa1504720. Epub 2015 Sep 17. N Engl J Med. 2015. PMID: 26378978 Clinical Trial. - Update on SGLT2 Inhibitors-New Data Released at the American Diabetes Association.
Lee S. Lee S. Crit Pathw Cardiol. 2017 Sep;16(3):93-95. doi: 10.1097/HPC.0000000000000125. Crit Pathw Cardiol. 2017. PMID: 28742644 Review. - Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis.
Wu JH, Foote C, Blomster J, Toyama T, Perkovic V, Sundström J, Neal B. Wu JH, et al. Lancet Diabetes Endocrinol. 2016 May;4(5):411-9. doi: 10.1016/S2213-8587(16)00052-8. Epub 2016 Mar 18. Lancet Diabetes Endocrinol. 2016. PMID: 27009625 Review.
Cited by
- Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases.
Bashier A, Bin Hussain A, Abdelgadir E, Alawadi F, Sabbour H, Chilton R. Bashier A, et al. Diabetol Metab Syndr. 2019 Sep 26;11:80. doi: 10.1186/s13098-019-0476-0. eCollection 2019. Diabetol Metab Syndr. 2019. PMID: 31572499 Free PMC article. Review. - SGLT2 Inhibitors: Cardiovascular Benefits Beyond HbA1c-Translating Evidence into Practice.
Ali A, Bain S, Hicks D, Newland Jones P, Patel DC, Evans M, Fernando K, James J, Milne N, Viljoen A, Wilding J; As part of The Improving Diabetes Steering Committee. Ali A, et al. Diabetes Ther. 2019 Oct;10(5):1595-1622. doi: 10.1007/s13300-019-0657-8. Epub 2019 Jul 9. Diabetes Ther. 2019. PMID: 31290126 Free PMC article. Review. - Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017.
Einarson TR, Acs A, Ludwig C, Panton UH. Einarson TR, et al. Cardiovasc Diabetol. 2018 Jun 8;17(1):83. doi: 10.1186/s12933-018-0728-6. Cardiovasc Diabetol. 2018. PMID: 29884191 Free PMC article. - SGLT2 inhibitors as add on therapy in type 2 diabetes: a real world study.
Tamez-Perez HE, Delgadillo-Esteban E, Soni-Duque D, Hernández-Coria MI, Tamez-Peña AL. Tamez-Perez HE, et al. J Diabetes Metab Disord. 2017 Jun 30;16:27. doi: 10.1186/s40200-017-0308-4. eCollection 2017. J Diabetes Metab Disord. 2017. PMID: 28680862 Free PMC article.
References
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) [webpage on the Internet] Guidance for Industry Diabetes Mellitus – Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. [Accessed February 14, 2016]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati....
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macroVascular events): a randomised controlled trial. Lancet. 2005;366(9493):1279–1289. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources