Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors) - PubMed (original) (raw)
Randomized Controlled Trial
. 2017 Jul 18;136(3):249-259.
doi: 10.1161/CIRCULATIONAHA.117.029190. Epub 2017 May 18.
Matthew A Cavender 2, Alex Z Fu 2, John P Wilding 2, Kamlesh Khunti 2, Reinhard W Holl 2, Anna Norhammar 2, Kåre I Birkeland 2, Marit Eika Jørgensen 2, Marcus Thuresson 2, Niki Arya 2, Johan Bodegård 2, Niklas Hammar 2, Peter Fenici 2; CVD-REAL Investigators and Study Group*
Affiliations
- PMID: 28522450
- PMCID: PMC5515629
- DOI: 10.1161/CIRCULATIONAHA.117.029190
Randomized Controlled Trial
Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors)
Mikhail Kosiborod et al. Circulation. 2017.
Abstract
Background: Reduction in cardiovascular death and hospitalization for heart failure (HHF) was recently reported with the sodium-glucose cotransporter-2 inhibitor (SGLT-2i) empagliflozin in patients with type 2 diabetes mellitus who have atherosclerotic cardiovascular disease. We compared HHF and death in patients newly initiated on any SGLT-2i versus other glucose-lowering drugs in 6 countries to determine if these benefits are seen in real-world practice and across SGLT-2i class.
Methods: Data were collected via medical claims, primary care/hospital records, and national registries from the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom. Propensity score for SGLT-2i initiation was used to match treatment groups. Hazard ratios for HHF, death, and their combination were estimated by country and pooled to determine weighted effect size. Death data were not available for Germany.
Results: After propensity matching, there were 309 056 patients newly initiated on either SGLT-2i or other glucose-lowering drugs (154 528 patients in each treatment group). Canagliflozin, dapagliflozin, and empagliflozin accounted for 53%, 42%, and 5% of the total exposure time in the SGLT-2i class, respectively. Baseline characteristics were balanced between the 2 groups. There were 961 HHF cases during 190 164 person-years follow-up (incidence rate, 0.51/100 person-years). Of 215 622 patients in the United States, Norway, Denmark, Sweden, and the United Kingdom, death occurred in 1334 (incidence rate, 0.87/100 person-years), and HHF or death in 1983 (incidence rate, 1.38/100 person-years). Use of SGLT-2i, versus other glucose-lowering drugs, was associated with lower rates of HHF (hazard ratio, 0.61; 95% confidence interval, 0.51-0.73; P<0.001); death (hazard ratio, 0.49; 95% confidence interval, 0.41-0.57; P<0.001); and HHF or death (hazard ratio, 0.54; 95% confidence interval, 0.48-0.60; P<0.001) with no significant heterogeneity by country.
Conclusions: In this large multinational study, treatment with SGLT-2i versus other glucose-lowering drugs was associated with a lower risk of HHF and death, suggesting that the benefits seen with empagliflozin in a randomized trial may be a class effect applicable to a broad population of patients with type 2 diabetes mellitus in real-world practice.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT02993614.
Keywords: canagliflozin; dapagliflozin; death; diabetes mellitus; empagliflozin; heart failure; sodium glucose transporter 2.
© 2017 The Authors.
Figures
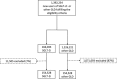
Figure 1.
Patient flow chart for all countries/databases combined. A large number of patients were excluded from the other GLD group because of the protocol mandated 1:1 match, and given the smaller number of patients in the SGLT-2i group. GLD indicates glucose-lowering drug; and SGLT-2i, sodium-glucose cotransporter-2 inhibitor.
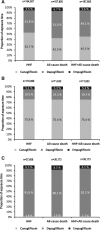
Figure 2.
Contribution of the SGLT-2 inhibitor class as a proportion of exposure time in the propensity-match cohorts. A, All countries combined. B, United States only. C, European countries combined. HHF indicates hospitalization for heart failure; and SGLT-2, sodium-glucose cotransporter-2.
Figure 3.
Hazard ratios and 95% CI for the outcome of HHF. A, On treatment, unadjusted. B, On treatment, adjusted (model adjusted for history of heart failure, age, sex, frailty, history of myocardial infarction, history of atrial fibrillation, hypertension, obesity/body mass index, duration of diabetes mellitus, ACE inhibitor or ARB use, β-blocker or α-blocker use, Ca2+ channel blocker use, loop diuretic use, thiazide diuretic use). C, Intent-to-treat, unadjusted. ACE indicates angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CI, confidence interval; CPRD, Clinical Practice Research Datalink; DPV, Diabetes Patientenverlaufsdokumentation (Diabetes Prospective Follow-Up); HHF, hospitalization for heart failure; oGLD, other glucose-lowering drugs; SGLT-2i, sodium-glucose cotransporter-2 inhibitor; and THIN, The Health Improvement Network.
Figure 4.
Hazard ratios and 95% CI for the outcome of all-cause death and composite of hospitalization for heart failure or all-cause death. A, All-cause death: on treatment, unadjusted. B, All-cause death: on treatment, adjusted (model adjusted for history of heart failure, age, sex, frailty, history of myocardial infarction, history of atrial fibrillation, hypertension, obesity/body mass index, duration of diabetes mellitus, ACE inhibitor or ARB use, β-blocker or α-blocker use, Ca2+ channel blocker use, loop diuretic use, thiazide diuretic use). C, All-cause death: intent-to-treat, unadjusted. D, Hospitalization for heart failure or all-cause death: on treatment, unadjusted. E, Hospitalization for heart failure or all-cause death: on treatment, adjusted (model adjusted for history of heart failure, age, sex, frailty, history of myocardial infarction, history of atrial fibrillation, hypertension, obesity/body mass index, duration of diabetes mellitus, ACE inhibitor or ARB use, β-blocker or α-blocker use, Ca2+ channel blocker use, loop diuretic use, thiazide diuretic use). F, Hospitalization for heart failure or all-cause death: intent-to-treat, unadjusted. ACE indicates angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CI, confidence interval; CPRD, Clinical Practice Research Datalink; DPV, Diabetes Patientenverlaufsdokumentation (Diabetes Prospective Follow-Up); oGLD, other glucose-lowering drugs; SGLT-2i, sodium-glucose cotransporter-2 inhibitor; and THIN, The Health Improvement Network.
Comment in
- Reality and Truth: Balancing the Hope and the Hype of Real-World Evidence.
Patel A, Billot L. Patel A, et al. Circulation. 2017 Jul 18;136(3):260-262. doi: 10.1161/CIRCULATIONAHA.117.029233. Circulation. 2017. PMID: 28716830 No abstract available. - Sodium-glucose cotransporter 2 inhibitors and death and heart failure in type 2 diabetes.
Yanai H. Yanai H. Ann Transl Med. 2017 Dec;5(23):470. doi: 10.21037/atm.2017.09.22. Ann Transl Med. 2017. PMID: 29285503 Free PMC article. No abstract available. - Comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors: SGLT2 inhibitors in the real world.
MacIsaac RJ, Ekinci EI. MacIsaac RJ, et al. Ann Transl Med. 2017 Dec;5(23):474. doi: 10.21037/atm.2017.10.11. Ann Transl Med. 2017. PMID: 29285507 Free PMC article. No abstract available. - Response by Kosiborod et al to Letters Regarding Article, "Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors)".
Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jørgensen ME, Thuresson M, Arya N, Bodegård J, Hammar N, Fenici P; CVD-REAL Investigators and Study Group. Kosiborod M, et al. Circulation. 2018 Feb 27;137(9):989-991. doi: 10.1161/CIRCULATIONAHA.117.031847. Circulation. 2018. PMID: 29483180 No abstract available. - Sodium-glucose cotransporter-2 inhibitors and cardiovascular outcomes: insights from the CVD-REAL study.
Saad M. Saad M. Ann Transl Med. 2018 Feb;6(3):55. doi: 10.21037/atm.2017.11.08. Ann Transl Med. 2018. PMID: 29610747 Free PMC article. No abstract available.
Similar articles
- Cardiovascular Events Associated With SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL 2 Study.
Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, Tangri N, Goh SY, Thuresson M, Chen H, Surmont F, Hammar N, Fenici P; CVD-REAL Investigators and Study Group. Kosiborod M, et al. J Am Coll Cardiol. 2018 Jun 12;71(23):2628-2639. doi: 10.1016/j.jacc.2018.03.009. Epub 2018 Mar 11. J Am Coll Cardiol. 2018. PMID: 29540325 Clinical Trial. - Cardiovascular outcomes with sodium-glucose cotransporter-2 inhibitors vs other glucose-lowering drugs in 13 countries across three continents: analysis of CVD-REAL data.
Khunti K, Kosiborod M, Kim DJ, Kohsaka S, Lam CSP, Goh SY, Chiang CE, Shaw JE, Cavender MA, Tangri N, Franch-Nadal J, Holl RW, Jørgensen ME, Norhammar A, Eriksson JG, Zaccardi F, Karasik A, Magliano DJ, Thuresson M, Chen H, Wittbrodt E, Bodegård J, Surmont F, Fenici P; CVD-REAL Investigators and Study Group. Khunti K, et al. Cardiovasc Diabetol. 2021 Jul 31;20(1):159. doi: 10.1186/s12933-021-01345-z. Cardiovasc Diabetol. 2021. PMID: 34332558 Free PMC article. - Cardiovascular Outcomes in Patients Initiating First-Line Treatment of Type 2 Diabetes With Sodium-Glucose Cotransporter-2 Inhibitors Versus Metformin : A Cohort Study.
Shin H, Schneeweiss S, Glynn RJ, Patorno E. Shin H, et al. Ann Intern Med. 2022 Jul;175(7):927-937. doi: 10.7326/M21-4012. Epub 2022 May 24. Ann Intern Med. 2022. PMID: 35605236 - Rationale for the Early Use of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes.
Handelsman Y. Handelsman Y. Adv Ther. 2019 Oct;36(10):2567-2586. doi: 10.1007/s12325-019-01054-w. Epub 2019 Aug 23. Adv Ther. 2019. PMID: 31444707 Free PMC article. Review.
Cited by
- Comparing ischemic cardiovascular effectiveness and safety between individual SGLT-2 inhibitors and DPP-4 inhibitors in patients with type 2 diabetes: a nationwide population-based cohort study.
Kim H, Seo JH, Nam JH, Lim Y, Choi KH, Kim K. Kim H, et al. Front Pharmacol. 2024 Oct 30;15:1443175. doi: 10.3389/fphar.2024.1443175. eCollection 2024. Front Pharmacol. 2024. PMID: 39545068 Free PMC article. - The effect of empagliflozin on circulating endothelial progenitor cells in patients with diabetes and stable coronary artery disease.
Hershenson R, Nardi-Agmon I, Leshem-Lev D, Kornowski R, Eisen A. Hershenson R, et al. Cardiovasc Diabetol. 2024 Oct 28;23(1):386. doi: 10.1186/s12933-024-02466-x. Cardiovasc Diabetol. 2024. PMID: 39468546 Free PMC article. - Empagliflozin Use Is Associated With Lower Risk of All-Cause Mortality, Hospitalization for Heart Failure, and End-Stage Renal Disease Compared to DPP-4i in Nordic Type 2 Diabetes Patients: Results From the EMPRISE (Empagliflozin Comparative Effectiveness and Safety) Study.
Langslet G, Nyström T, Vistisen D, Carstensen B, Grip ET, Casajust P, Tskhvarashvili G, Hoti F, Klement R, Karlsdotter K, Tuovinen M, Ofstad AP, Lajer M, Shay C, Koeneman L, Farsani SF, Niskanen L, Halvorsen S. Langslet G, et al. J Diabetes Res. 2024 Oct 12;2024:6142211. doi: 10.1155/2024/6142211. eCollection 2024. J Diabetes Res. 2024. PMID: 39430801 Free PMC article. - FOUNTAIN: a modular research platform for integrated real-world evidence generation.
Oberprieler NG, Pladevall-Vila M, Johannes C, Layton JB, Golozar A, Lavallee M, Liu F, Kubin M, Vizcaya D. Oberprieler NG, et al. BMC Med Res Methodol. 2024 Oct 1;24(1):224. doi: 10.1186/s12874-024-02344-w. BMC Med Res Methodol. 2024. PMID: 39354358 Free PMC article. - Advances in understanding and managing pediatric heart failure and transplant.
Xu W, Richmond M. Xu W, et al. Curr Opin Pediatr. 2024 Oct 1;36(5):489-495. doi: 10.1097/MOP.0000000000001393. Epub 2024 Aug 12. Curr Opin Pediatr. 2024. PMID: 39254752 Review.
References
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. - PMC - PubMed
- Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; Functional Genomics and Translational Biology Interdisciplinary Working Group. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. - PubMed
- Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, O’Keeffe LM, Gao P, Wood AM, Burgess S, Freitag DF, Pennells L, Peters SA, Hart CL, Håheim LL, Gillum RF, Nordestgaard BG, Psaty BM, Yeap BB, Knuiman MW, Nietert PJ, Kauhanen J, Salonen JT, Kuller LH, Simons LA, van der Schouw YT, Barrett-Connor E, Selmer R, Crespo CJ, Rodriguez B, Verschuren WM, Salomaa V, Svärdsudd K, van der Harst P, Björkelund C, Wilhelmsen L, Wallace RB, Brenner H, Amouyel P, Barr EL, Iso H, Onat A, Trevisan M, D’Agostino RB, Sr, Cooper C, Kavousi M, Welin L, Roussel R, Hu FB, Sato S, Davidson KW, Howard BV, Leening MJ, Leening M, Rosengren A, Dörr M, Deeg DJ, Kiechl S, Stehouwer CD, Nissinen A, Giampaoli S, Donfrancesco C, Kromhout D, Price JF, Peters A, Meade TW, Casiglia E, Lawlor DA, Gallacher J, Nagel D, Franco OH, Assmann G, Dagenais GR, Jukema JW, Sundström J, Woodward M, Brunner EJ, Khaw KT, Wareham NJ, Whitsel EA, Njølstad I, Hedblad B, Wassertheil-Smoller S, Engström G, Rosamond WD, Selvin E, Sattar N, Thompson SG, Danesh J Emerging Risk Factors Collaboration. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. - PMC - PubMed
- Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. - PubMed
- IDF Diabetes Atlas Group. Update of mortality attributable to diabetes for the IDF diabetes atlas: estimates for the year 2011. Diabetes Res Clin Pract. 2013;100:277–279. doi: 10.1016/j.diabres.2013.02.005. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical