The GRADE Working Group clarifies the construct of certainty of evidence - PubMed (original) (raw)
Practice Guideline
doi: 10.1016/j.jclinepi.2017.05.006. Epub 2017 May 18.
David Rind 2, Elie A Akl 3, Shaun Treweek 4, Reem A Mustafa 5, Alfonso Iorio 6, Brian S Alper 7, Joerg J Meerpohl 8, M Hassan Murad 9, Mohammed T Ansari 10, Srinivasa Vittal Katikireddi 11, Pernilla Östlund 12, Sofia Tranæus 13, Robin Christensen 14, Gerald Gartlehner 15, Jan Brozek 6, Ariel Izcovich 16, Holger Schünemann 6, Gordon Guyatt 6
Affiliations
- PMID: 28529184
- PMCID: PMC6542664
- DOI: 10.1016/j.jclinepi.2017.05.006
Practice Guideline
The GRADE Working Group clarifies the construct of certainty of evidence
Monica Hultcrantz et al. J Clin Epidemiol. 2017 Jul.
Abstract
Objective: To clarify the grading of recommendations assessment, development and evaluation (GRADE) definition of certainty of evidence and suggest possible approaches to rating certainty of the evidence for systematic reviews, health technology assessments, and guidelines.
Study design and setting: This work was carried out by a project group within the GRADE Working Group, through brainstorming and iterative refinement of ideas, using input from workshops, presentations, and discussions at GRADE Working Group meetings to produce this document, which constitutes official GRADE guidance.
Results: Certainty of evidence is best considered as the certainty that a true effect lies on one side of a specified threshold or within a chosen range. We define possible approaches for choosing threshold or range. For guidelines, what we call a fully contextualized approach requires simultaneously considering all critical outcomes and their relative value. Less-contextualized approaches, more appropriate for systematic reviews and health technology assessments, include using specified ranges of magnitude of effect, for example, ranges of what we might consider no effect, trivial, small, moderate, or large effects.
Conclusion: It is desirable for systematic review authors, guideline panelists, and health technology assessors to specify the threshold or ranges they are using when rating the certainty in evidence.
Keywords: Certainty of evidence; GRADE; Guidelines; Health technology assessment; Systematic reviews; Thresholds.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
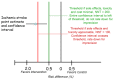
Figure 1. Rating certainty that the true effect lies in a particular range: an illustration from previous GRADE writings (2)
Comment in
- The construct of certainty of evidence has not been disseminated to systematic reviews and clinical practice guidelines; response to 'The GRADE Working Group' et al.
Taito S, Tsujimoto Y, Kataoka Y. Taito S, et al. J Clin Epidemiol. 2022 Jul;147:171-172. doi: 10.1016/j.jclinepi.2022.03.013. Epub 2022 Mar 24. J Clin Epidemiol. 2022. PMID: 35341947 No abstract available.
Similar articles
- GRADE guidelines 32: GRADE offers guidance on choosing targets of GRADE certainty of evidence ratings.
Zeng L, Brignardello-Petersen R, Hultcrantz M, Siemieniuk RAC, Santesso N, Traversy G, Izcovich A, Sadeghirad B, Alexander PE, Devji T, Rochwerg B, Murad MH, Morgan R, Christensen R, Schünemann HJ, Guyatt GH. Zeng L, et al. J Clin Epidemiol. 2021 Sep;137:163-175. doi: 10.1016/j.jclinepi.2021.03.026. Epub 2021 Apr 20. J Clin Epidemiol. 2021. PMID: 33857619 - Defining ranges for certainty ratings of diagnostic accuracy: a GRADE concept paper.
Hultcrantz M, Mustafa RA, Leeflang MMG, Lavergne V, Estrada-Orozco K, Ansari MT, Izcovich A, Singh J, Chong LY, Rutjes A, Steingart K, Stein A, Sekercioglu N, Arevalo-Rodriguez I, Morgan RL, Guyatt G, Bossuyt P, Langendam MW, Schünemann HJ. Hultcrantz M, et al. J Clin Epidemiol. 2020 Jan;117:138-148. doi: 10.1016/j.jclinepi.2019.05.002. Epub 2019 May 18. J Clin Epidemiol. 2020. PMID: 31112801 - GRADE guidance 35: update on rating imprecision for assessing contextualized certainty of evidence and making decisions.
Schünemann HJ, Neumann I, Hultcrantz M, Brignardello-Petersen R, Zeng L, Murad MH, Izcovich A, Morgano GP, Baldeh T, Santesso N, Cuello CG, Mbuagbaw L, Guyatt G, Wiercioch W, Piggott T, De Beer H, Vinceti M, Mathioudakis AG, Mayer MG, Mustafa R, Filippini T, Iorio A, Nieuwlaat R, Marcucci M, Coello PA, Bonovas S, Piovani D, Tomlinson G, Akl EA; GRADE Working Group. Schünemann HJ, et al. J Clin Epidemiol. 2022 Oct;150:225-242. doi: 10.1016/j.jclinepi.2022.07.015. Epub 2022 Aug 5. J Clin Epidemiol. 2022. PMID: 35934266 - When applying GRADE, how do we decide the target of certainty of evidence rating?
Zeng L, Brignardello-Petersen R, Guyatt G. Zeng L, et al. Evid Based Ment Health. 2021 Jun 14;24(3):121-3. doi: 10.1136/ebmental-2020-300170. Online ahead of print. Evid Based Ment Health. 2021. PMID: 34127510 Free PMC article. Review. - [How to interpret the certainty of evidence based on GRADE (Grading of Recommendations, Assessment, Development and Evaluation)].
Schwingshackl L, Rüschemeyer G, Meerpohl JJ. Schwingshackl L, et al. Urologe A. 2021 Apr;60(4):444-454. doi: 10.1007/s00120-021-01471-2. Epub 2021 Feb 23. Urologe A. 2021. PMID: 33620513 Review. German.
Cited by
- Exploring the Effects of Qigong, Tai Chi, and Yoga on Fatigue, Mental Health, and Sleep Quality in Chronic Fatigue and Post-COVID Syndromes: A Systematic Review with Meta-Analysis.
Fricke-Comellas H, Heredia-Rizo AM, Casuso-Holgado MJ, Salas-González J, Fernández-Seguín LM. Fricke-Comellas H, et al. Healthcare (Basel). 2024 Oct 11;12(20):2020. doi: 10.3390/healthcare12202020. Healthcare (Basel). 2024. PMID: 39451436 Free PMC article. Review. - Effects of lower-limb active resistance exercise on mobility, physical function, knee strength and pain intensity in patients with total knee arthroplasty: a systematic review and meta-analysis.
Wei G, Shang Z, Li Y, Wu Y, Zhang L. Wei G, et al. BMC Musculoskelet Disord. 2024 Sep 12;25(1):730. doi: 10.1186/s12891-024-07845-9. BMC Musculoskelet Disord. 2024. PMID: 39267026 Free PMC article. - Immunomodulators and immunosuppressants for progressive multiple sclerosis: a network meta-analysis.
Ridley B, Minozzi S, Gonzalez-Lorenzo M, Del Giovane C, Piggott T, Filippini G, Peryer G, Foschi M, Tramacere I, Baldin E, Nonino F. Ridley B, et al. Cochrane Database Syst Rev. 2024 Sep 10;9(9):CD015443. doi: 10.1002/14651858.CD015443.pub2. Cochrane Database Syst Rev. 2024. PMID: 39254048 Free PMC article. - Effects of cocoa consumption on cardiometabolic risk markers: Protocol for a systematic review and meta-analysis of randomized controlled trials.
Arisi TOP, da Silva DS, Stein E, Weschenfelder C, de Oliveira PC, Marcadenti A, Lehnen AM, Waclawovsky G. Arisi TOP, et al. PLoS One. 2024 Sep 9;19(9):e0309824. doi: 10.1371/journal.pone.0309824. eCollection 2024. PLoS One. 2024. PMID: 39250491 Free PMC article. - Research Quality of Clinical Trials Reported for Foods with Function Claims in Japan, 2023-2024: Evaluation Based on a Revised Tool to Assess Risk of Bias in Randomized Trials.
Kamioka H, Kitayuguchi J, Origasa H, Tsutani K. Kamioka H, et al. Nutrients. 2024 Aug 17;16(16):2744. doi: 10.3390/nu16162744. Nutrients. 2024. PMID: 39203880 Free PMC article. Review.
References
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. - PubMed
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol. 2011;64(12):1283–93. - PubMed
- Schunemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? J Clin Epidemiol. 2016;75:6–15. - PubMed
- Schunemann HJ. Guidelines 2.0: do no net harm-the future of practice guideline development in asthma and other diseases. Curr Allergy Asthma Rep. 2011;11(3):261–8. - PubMed
Publication types
MeSH terms
Grants and funding
- MC_UU_12017/13/MRC_/Medical Research Council/United Kingdom
- HSRU2/CSO_/Chief Scientist Office/United Kingdom
- SPHSU13/CSO_/Chief Scientist Office/United Kingdom
- SCAF/15/02/CSO_/Chief Scientist Office/United Kingdom
- MC_UU_12017/15/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous