Mechanisms of DNA replication termination - PubMed (original) (raw)
Review
. 2017 Aug;18(8):507-516.
doi: 10.1038/nrm.2017.42. Epub 2017 May 24.
Affiliations
- PMID: 28537574
- PMCID: PMC6386472
- DOI: 10.1038/nrm.2017.42
Review
Mechanisms of DNA replication termination
James M Dewar et al. Nat Rev Mol Cell Biol. 2017 Aug.
Abstract
Genome duplication is carried out by pairs of replication forks that assemble at origins of replication and then move in opposite directions. DNA replication ends when converging replication forks meet. During this process, which is known as replication termination, DNA synthesis is completed, the replication machinery is disassembled and daughter molecules are resolved. In this Review, we outline the steps that are likely to be common to replication termination in most organisms, namely, fork convergence, synthesis completion, replisome disassembly and decatenation. We briefly review the mechanism of termination in the bacterium Escherichia coli and in simian virus 40 (SV40) and also focus on recent advances in eukaryotic replication termination. In particular, we discuss the recently discovered E3 ubiquitin ligases that control replisome disassembly in yeast and higher eukaryotes, and how their activity is regulated to avoid genome instability.
Figures
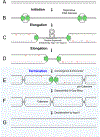
Figure 1:. Steps in DNA replication
Generic illustration of replication initiation (A-B), elongation (C-D), and five events that are unique to replication termination (D-G). The replicative DNA helicase is depicted without reference to a specific translocation mechanism; RNA primers are in red. The order of the termination events is hypothetical.
Figure 2:. Replication termination in Escherichia coli
(A) Depiction of the Escherichia coli chromosome, including the origin of replication oriC, and the ten ter sites shown as red and blue arrowheads. The termination zone is underlined in red. In the box, the green arrow shows a replication fork passing through a ter site in the permissive orientation, and the red arrow shows a fork stalling at a ter site in the non-permissive orientation. (B) Two scenarios of fork stalling in the termination zone. (a) The rightward fork (Fork 1) arrives first and stalls at terC, followed by the arrival of the leftward (Fork 2). (b) The two forks arrive to the termination zone contemporaneously and meet between terC and terA. (C) Possible mechanism of E. coli replication termination. (a) Forks converge between ter sites with the formation of pre-catenanes. (b) Two DNA synthesis protein B (DnaB) replicative helicase complexes pass each other and collide with the downstream leading strand, generating a 3’ flap. DnaB dissociates, the 3’ flap is removed, gaps are filled in and the final Okazaki fragment is processed by DNA polymerase I (Pol I). (c) Nicks are ligated and the final catenane, which is generated during the completion of DNA synthesis is removed (not shown). (D) Possible mechanism of replication re-initiation. (a) If the 3’ flaps are not removed or remodeled, a new replication fork is established, which prevents the completion of termination. (b) The free end re-invades the sister chromatid using recombination protein A (RecA) and RecBCD, which establish a new replication fork. (c) The Holliday junction is resolved and DnaB is re-loaded onto the fork by primosomal proteins A (PriA) and PriB.
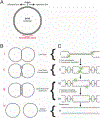
Figure 3:. Model for simian virus 40 DNA replication termination
(A) The simian virus 40 (SV40) chromosome is a plasmid that includes the origin of replication and termination zone (underlined in red). (B) Late stages of SV40 DNA replication. (a) Late theta intermediate, when fork convergence begins. (b) Fork convergence, when superhelical stress is dissipated by the formation of pre-catenanes. (c) Catenated dimers are generated when pre-catenanes are converted to catenanes at the end of replication. (d) Decatenation produces two circular monomers. (C) Hypothetical mechanism of SV40 replication termination. (a) When forks come within 450 bp of each other, they stall, possibly owing to reduced formation of pre-catenanes. (b) Forks converge, leading to the formation of pre-catenanes and the encounter of the large T antigen (T-ag) helicases. Whether the helicases stall upon encounter is not known. (c) The helicases pass each other and stall at the downstream Okazaki fragment, accounting for the single-stranded DNA gap that persists on replicated molecules. (d) T-ag is unloaded, the remaining gaps are filled in. (e) The catenanes are removed, yielding fully replicated and decatenated daughter chromosomes.
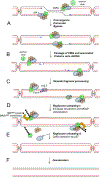
Figure 4:. Model of eukaryotic replication termination
(A) After copying most of the replicon, forks come too close to each other to allow formation of supercoils in the unreplicated DNA, leading to the onset of convergence. During convergence, which lasts until forks encounter each other, topological stress is relieved by the formation of precatenanes. An end-on view of CMG illustrates the presence of single-stranded DNA in its central channel. (B) The encounter causes no detectable fork stalling, implying that converging CMGs bypass each other. After bypass, CMG helicases keep translocating until they reach a downstream Okazaki fragment. (C) The CMG helicases pass over the ssDNA–dsDNA junction and keep moving on dsDNA (see end-on view). (D) The leading strand is extended to the downstream Okazaki fragment. The last Okazaki fragment is processed, possibly by de novo recruitment of DNA polymerase δ (Pol δ) and by 3’ flap processing by flap endonuclease 1 (FEN1). (E) Once CMG encircles dsDNA, it undergoes polyubiquitylation on its MCM7 subunit by SCFDia2 or CRL2Lrr1. The ubiquitylated MCM7 is extracted from chromatin by the ATPase p97 ATPase. (F) Catenanes are removed. CMG,
C
DC45–
M
CM–
G
INS
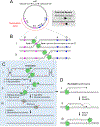
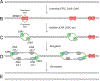
Box 1:. Eukaryotic replication initiation and elongation: the basics
Here we provide a brief summary of eukaryotic DNA replication initiation and elongation (reviewed in ,,,). ‘Licensing’ of DNA replication occurs in the G1 phase of the cell cycle, when the origin recognition complex (ORC), the ATPase cell division cycle 6 (CDC6) and CDC10 dependent transcript 1 (CDT1) cooperate to recruit two minichromosome maintenance 2–7 (MCM2–7) complexes to each origin of replication, thereby forming the pre-replicative complex (pre-RC) (see the figure). MCM2–7 is a heterohexamer composed of the related ATPases MCM2–MCM7 and serves as the motor of the replicative helicase. Within pre-RCs, two inactive MCM2–7 complexes encircle double-stranded DNA, with their N-terminal tiers oriented towards each other to form a tight dimer interface. In S phase, a subset of pre-RCs undergoes activation by cyclin-dependent kinase (CDK), Dbf4-dependent kinase (DDK) and many accessory factors, leading to the binding of two helicase co-factors, CDC45 and the four-subunit Go-Ichi-Ni-San (GINS) complex, to each MCM2–7 complex, thereby forming the active CDC45–MCM–GINS (CMG) helicase (see the figure). CMG encircles the leading strand and translocates along it in the 3’ to 5’ direction; the trailing edge of CMG is formed by the C-terminal lobe of MCM2–7. CMG unwinds the origin, allowing the assembly of two DNA replication forks that travel away from the origin. Cells prevent re-replication by blocking licensing in S phase. In yeasts, although origin sequences are well-defined, initiation is partly stochastic, so that the program of origin firing is probably unique in every cell. In higher eukaryotes, the DNA sequences of origins are poorly defined and initiation is inefficient and frequently occurs in large zones, resulting in replication programs that are even more stochastic than in yeasts. The replisome is a macromolecular assembly composed of multiple protein complexes. The leading strand is synthesized continuously by DNA polymerase ε (Pol ε), whereas the lagging strand is composed of Okazaki fragments synthesized by Pol δ (see the figure). Pol δ acquires processivity by its association with the ring-shaped protein proliferating cell nuclear antigen, which is deposited around DNA by replication factor C. The leading strand and every Okazaki fragment are primed by Pol α−primase, which synthesizes a ~10 nucleotide RNA primer and then extends it by 20–30 nucleotides of DNA before the switch to the more processive Pol ε or Pol δ occurs. When the 3’ end of one Okazaki fragment reaches the 5’ of another, Pol δ performs strand displacement synthesis (Figure 4D). The resulting flap is removed by flap endonuclease 1. Long flaps are degraded by the helicase-nuclease DNA synthesis defective protein 2. The replisome also contains topoisomerase I, chromatin remodeling factors, checkpoint signaling proteins and cohesion establishment factors. CMG binds directly to Pol ε and indirectly to Pol α through chromosome transmission fidelity protein 4. Therefore, unlike in bacteria, leading and lagging strand polymerases appear not to form a stable complex in eukaryotes.
Similar articles
- Pif1-Family Helicases Support Fork Convergence during DNA Replication Termination in Eukaryotes.
Deegan TD, Baxter J, Ortiz Bazán MÁ, Yeeles JTP, Labib KPM. Deegan TD, et al. Mol Cell. 2019 Apr 18;74(2):231-244.e9. doi: 10.1016/j.molcel.2019.01.040. Epub 2019 Mar 5. Mol Cell. 2019. PMID: 30850330 Free PMC article. - Termination of DNA replication forks: "Breaking up is hard to do".
Bailey R, Priego Moreno S, Gambus A. Bailey R, et al. Nucleus. 2015;6(3):187-96. doi: 10.1080/19491034.2015.1035843. Epub 2015 Apr 2. Nucleus. 2015. PMID: 25835602 Free PMC article. - Random and site-specific replication termination.
Dalgaard JZ, Eydmann T, Koulintchenko M, Sayrac S, Vengrova S, Yamada-Inagawa T. Dalgaard JZ, et al. Methods Mol Biol. 2009;521:35-53. doi: 10.1007/978-1-60327-815-7_3. Methods Mol Biol. 2009. PMID: 19563100 Review. - Stalled replication forks: making ends meet for recognition and stabilization.
Masai H, Tanaka T, Kohda D. Masai H, et al. Bioessays. 2010 Aug;32(8):687-97. doi: 10.1002/bies.200900196. Bioessays. 2010. PMID: 20658707 Review. - The Escherichia coli Tus-Ter replication fork barrier causes site-specific DNA replication perturbation in yeast.
Larsen NB, Sass E, Suski C, Mankouri HW, Hickson ID. Larsen NB, et al. Nat Commun. 2014 Apr 7;5:3574. doi: 10.1038/ncomms4574. Nat Commun. 2014. PMID: 24705096
Cited by
- USP37 prevents unscheduled replisome unloading through MCM complex deubiquitination.
Bolhuis DL, Fleifel D, Bonacci T, Wang X, Mouery BL, Cook JG, Brown NG, Emanuele MJ. Bolhuis DL, et al. bioRxiv [Preprint]. 2024 Sep 3:2024.09.03.610997. doi: 10.1101/2024.09.03.610997. bioRxiv. 2024. PMID: 39282338 Free PMC article. Preprint. - USP37 prevents premature disassembly of stressed replisomes by TRAIP.
Kochenova OV, D'Alessandro G, Pilger D, Schmid E, Richards SL, Garcia MR, Jhujh SS, Voigt A, Gupta V, Carnie CJ, Wu RA, Gueorguieva N, Stewart GS, Walter JC, Jackson SP. Kochenova OV, et al. bioRxiv [Preprint]. 2024 Sep 4:2024.09.03.611025. doi: 10.1101/2024.09.03.611025. bioRxiv. 2024. PMID: 39282314 Free PMC article. Preprint. - YIPF2 regulates genome integrity.
Zhang X, Wang T. Zhang X, et al. Cell Biosci. 2024 Sep 5;14(1):114. doi: 10.1186/s13578-024-01300-x. Cell Biosci. 2024. PMID: 39238039 Free PMC article. - DNA replication recruits a friend to overcome a challenging break-up.
Dewar JM. Dewar JM. EMBO J. 2024 Sep;43(18):3815-3817. doi: 10.1038/s44318-024-00204-3. Epub 2024 Aug 21. EMBO J. 2024. PMID: 39169154 Free PMC article. - E3 ligases: a ubiquitous link between DNA repair, DNA replication and human disease.
Chauhan AS, Jhujh SS, Stewart GS. Chauhan AS, et al. Biochem J. 2024 Jul 17;481(14):923-944. doi: 10.1042/BCJ20240124. Biochem J. 2024. PMID: 38985307 Free PMC article. Review.
References
- Berezney R, Dubey DD & Huberman JA Heterogeneity of eukaryotic replicons, replicon clusters, and replication foci. Chromosoma 108, 471–484, (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases