Changes observed in erythrocyte cells exposed to an alternating current - PubMed (original) (raw)
Changes observed in erythrocyte cells exposed to an alternating current
Ionut Isaia Jeican et al. Clujul Med. 2017.
Abstract
Background and aims: Appliance of electric pulses induces red blood cells (RBCs) membrane poration, membrane aminophospholipid perturbation and alteration of the normal flip-flop process, resulting in various shape changes of the RBCs. We studied morphological and water permeability changes of RBCs bombarded with electrons in an alternating current circuit.
Methods: We used three venous blood samples of 100 mL and an alternating current device. The harvested blood was divided into four experimental sets to be used for various exposure times: 0 hours (control RBCs), 0.5h, 3h and 6h (electric-stimulated RBCs). Following the electric current each of the four sets were further divided into three samples: one for the assessment of the echinocytes/RBCs ratio, another for the electron microscopy study of ultrastructural changes induced by the alternating electrical current and a larger third one for determining water permeability of RCBs by 1H-NMR spectroscopy and morphological measurements.
Results: There is a small but statistically significant effect of the RBC exposure to alternating electric current on cell diameters. Exposure to electric current is positively and strongly correlated with the percentage of echinocytes in a duration-dependent manner. There is a strong and statistically significant correlation between electric current exposure and permeability to water as measured by 1H-NMR spectroscopy.
Conclusion: Following interactions between electric current and RBC membrane, certain modifications were observed in the erythrocyte structure. We attribute the increased cell size to a higher permeability to water and a decreased tonicity. This leads to the transformation of the RBCs into echinocytes.
Keywords: alternating current; echinocyte; erythrocytes; water permeability.
Figures
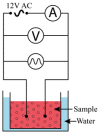
Figure 1
Schematic representation of the device used for the study of RBCs subjected to alternating current.
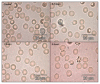
Figure 2
Native microscopic specimens images of control and 0.5 hours, 3 hours and 6 hours samples; objective: 100×. Bar: 20 μm.
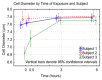
Figure 3
Influence of current exposure times on RBC diameter. Cell diameters in μm; exposure times in hours for the three subjects (see methods). Time 0 is considered as control.
Figure 4
Blood smears May-Grünwald-Giemsa images of control and 0.5 hours, 3 hours and 6 hours samples; objective: 100×. Bar: 20 μm.
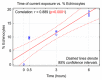
Figure 5
Scatterplot of echinocytes % vs. exposure times and fitted regression line (solid red line) with a 95% confidence interval (dashed red lines). Each point represents the percent of echinocytes resulting after exposure to electrical current, for each of the 3 subjects.
Figure 6
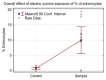
Differences in adjusted mean % of echinocytes between exposed and control.
Figure 7
Scanning electron microscope images of RBCs. Bar: 3 μm A. control, and exposure time of B. 0.5 hours, C. 3 hours, D. 6 hours exposure. Bar: 3μm.
Figure 8
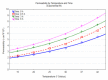
Influence of temperature and time on permeability
Similar articles
- Comparative nuclear magnetic resonance studies of water permeability of red blood cells from maternal venous blood and newborn umbilical cord blood.
Benga G, Frenţescu L, Matei H, Tigan S. Benga G, et al. Clin Chem Lab Med. 2001 Jul;39(7):606-11. doi: 10.1515/CCLM.2001.096. Clin Chem Lab Med. 2001. PMID: 11522105 - Comparative NMR studies of diffusional water permeability of red blood cells from different species: XVI Dingo (Canis familiaris dingo) and dog (Canis familiaris).
Benga G, Chapman BE, Matei H, Cox GC, Romeo T, Mironescu E, Kuchel PW. Benga G, et al. Cell Biol Int. 2010 Mar 8;34(4):373-8. doi: 10.1042/CBI20090006. Cell Biol Int. 2010. PMID: 19947930 - Early stage shape change of human erythrocytes after application of electric field pulses.
Baumann M. Baumann M. Mol Membr Biol. 2001 Apr-Jun;18(2):153-60. doi: 10.1080/09687680110034863. Mol Membr Biol. 2001. PMID: 11463207 - Diffusional water permeability of mammalian red blood cells.
Benga G, Borza T. Benga G, et al. Comp Biochem Physiol B Biochem Mol Biol. 1995 Dec;112(4):653-9. doi: 10.1016/0305-0491(95)00116-6. Comp Biochem Physiol B Biochem Mol Biol. 1995. PMID: 8590380 Review. - Light and Scanning Electron Microscopy of Red Blood Cells From Humans and Animal Species Providing Insights into Molecular Cell Biology.
Benga G, Cox G. Benga G, et al. Front Physiol. 2022 Jul 1;13:838071. doi: 10.3389/fphys.2022.838071. eCollection 2022. Front Physiol. 2022. PMID: 35845990 Free PMC article. Review.
Cited by
- Hump-Nosed Pit Viper (Hypnale hypnale) Venom-Induced Irreversible Red Blood Cell Aggregation, Inhibition by Monovalent Anti-Venom and N-Acetylcysteine.
Sandesha VD, Naveen P, Manikanta K, Mahalingam SS, Girish KS, Kemparaju K. Sandesha VD, et al. Cells. 2024 Jun 7;13(12):994. doi: 10.3390/cells13120994. Cells. 2024. PMID: 38920625 Free PMC article.
References
- Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175(4023):720–731. - PubMed
- Benga G. Water transport in red blood cell membranes. Prog Biophys Mol Biol. 1988;51(3):193–245. - PubMed
- Benga G. Water exchange through the erythrocyte membrane. Int Rev Cytol. 1989;114:273–316. - PubMed
- Smith BL, Agre P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J Biol Chem. 1991;266(10):6407–6415. - PubMed
- Agre P, Sasaki S, Chrispeels MJ. Aquaporins: a family of water channel protein. Am J Physiol. 1993;265(3 Pt 2):F461. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources