Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief - PubMed (original) (raw)
. 2017 May 31;9(392):eaal3447.
doi: 10.1126/scitranslmed.aal3447.
TinaMarie Lieu 1 2, Michelle L Halls 1, Nicholas A Veldhuis 1 2, Wendy L Imlach 3, Quynh N Mai 1 2, Daniel P Poole 1 2, Tim Quach 1 2, Luigi Aurelio 1 2, Joshua Conner 1 2, Carmen Klein Herenbrink 1 2, Nicholas Barlow 1, Jamie S Simpson 1, Martin J Scanlon 1, Bimbil Graham 1, Adam McCluskey 4, Phillip J Robinson 5, Virginie Escriou 6, Romina Nassini 7, Serena Materazzi 7, Pierangelo Geppetti 7, Gareth A Hicks 8, Macdonald J Christie 3, Christopher J H Porter 9 2, Meritxell Canals 9 2, Nigel W Bunnett 9 2 10 11
Affiliations
- PMID: 28566424
- PMCID: PMC6034632
- DOI: 10.1126/scitranslmed.aal3447
Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief
Dane D Jensen et al. Sci Transl Med. 2017.
Abstract
Typically considered to be cell surface sensors of extracellular signals, heterotrimeric GTP-binding protein (G protein)-coupled receptors (GPCRs) control many pathophysiological processes and are the target of 30% of therapeutic drugs. Activated receptors redistribute to endosomes, but researchers have yet to explore whether endosomal receptors generate signals that control complex processes in vivo and are viable therapeutic targets. We report that the substance P (SP) neurokinin 1 receptor (NK1R) signals from endosomes to induce sustained excitation of spinal neurons and pain transmission and that specific antagonism of the NK1R in endosomes with membrane-anchored drug conjugates provides more effective and sustained pain relief than conventional plasma membrane-targeted antagonists. Pharmacological and genetic disruption of clathrin, dynamin, and β-arrestin blocked SP-induced NK1R endocytosis and prevented SP-stimulated activation of cytosolic protein kinase C and nuclear extracellular signal-regulated kinase, as well as transcription. Endocytosis inhibitors prevented sustained SP-induced excitation of neurons in spinal cord slices in vitro and attenuated nociception in vivo. When conjugated to cholestanol to promote endosomal targeting, NK1R antagonists selectively inhibited endosomal signaling and sustained neuronal excitation. Cholestanol conjugation amplified and prolonged the antinociceptive actions of NK1R antagonists. These results reveal a critical role for endosomal signaling of the NK1R in the complex pathophysiology of pain and demonstrate the use of endosomally targeted GPCR antagonists.
Copyright © 2017, American Association for the Advancement of Science.
Conflict of interest statement
Competing interests: Work at N.W.B.’s laboratory was funded, in part, by Takeda Pharmaceuticals Inc. N.W.B. has filed a patent for use of lipidation to target GPCRs in endosomes. All other authors declare that they have no competing interests.
Figures
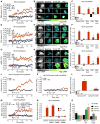
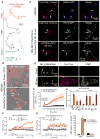
Fig. 1. N1R endocytosis-dependent compartmentalized signaling
(A to I) Effect of inhibitors of dynamin (Dy4) and clathrin (PS2), and of inactive (inact) analogs, on SP-induced spatiotemporal signaling profiles for nuclear ERK (NucEKAR) (A to C), cytosolic PKC (CytoCKAR) (D to F), and cytosolic cAMP (CytoEpac2) (G to I) measured in HEK293 cells using FRET biosensors. (A, D, and G) Time course of responses. (B, E, and H). Representative ratiometric images and sensor localization. Max, response to positive controls. Yellow arrows denote localization of FRET sensor and white arrows show the SP-stimulated signals in control cells and cells treated with Dy4 inact. (C, F, and I) Area under the curve (AUC) of (A), (D), and (G). (J and K) Effect of dynamin WT (J) or dominant negative K44E (K) overexpression on the spatiotemporal profile of SP-induced nuclear ERK. (L) AUC of (J) and (K). (M) Effect of clathrin heavy chain and dynamin-1 siRNA on the spatiotemporal profile of SP-induced nuclear ERK. (N) AUC of (M). (O) Effect of dynamin WT or K44E overexpression on the SP-induced SRE-SEAP. *P < 0.05, **P < 0.01, ***P < 0.001, vehicle (Veh); ^^P < 0.01, ^^^P < 0.001, control to inhibitors. (A to N) Thirty to 354 cells, three to five experiments. (O) n = 3 experiments. ANOVA, Tukey’s test (C, F, I, and N); Sidak’s test (L); Dunnett’s test (O).
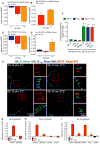
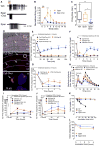
Fig. 2. G protein–dependent NK1R signaling in endosomes
(A to D) SP-induced BRET between NK1R-RLUC8 and KRAS-Venus (A) or RAB5A-Venus (B) and between Gαq-RLUC8 and Gγ2-Venus (C) or RAB5A-Venus (D) in HEK293 cells. *P < 0.05, **P < 0.01, ***P < 0.001 to baseline. Triplicate observations, n ≥ 3 experiments. (E) Localization of NK1R-IR (green), Gαq-IR (cyan), and EEA1-IR (red) in HEK293 cells by super-resolution microscopy. Blue boxes, plasma membrane; red boxes, endosomes. (F) Quantification of the proportion of endosomes containing NK1R-IR and Gαq-IR. Sixty to 66 cells per condition (20 to 22 cells from n = 3 experiments). ****P < 0.0001. (G to I) Effect of inhibitors of Gαq (UBO-QIC) or PLC (U73122) and Ca2+ chelation (EGTA) or inhibitors of Gαs (NF449) or PKC (GF109203X, GFX) on SP-induced nuclear ERK (G), cytosolic PKC (H), and cytosolic cAMP (I) measured using FRET biosensors. ***P < 0.001, SP to vehicle; ^^^P < 0.001, control to inhibitor. Thirty-five to 67 cells, three experiments. ANOVA, Dunnett’s test (A to D); Sidak’s test (F and G); Tukey’s test (H and I).
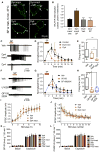
Fig. 3. NK1R endocytosis and neuronal excitation in spinal cord slices
(A) Effect of Dy4 and Dy4 inact on SP-induced endocytosis of NK1R-IR in rat spinal neurons. Arrows, internalized; arrowheads, cell surface NK1R. (B) Quantification of endocytosis. ****P < 0.0001. Eighteen neurons per group (six neurons in slices from n = 3 rats). (C to H) Effects of Dy4, Dy4 inact, U0126 (MEK inhibitor), and GF109203X (PKC inhibitor) on SP-induced firing of rat spinal neurons. (C and F) Representative traces. (D and G) Firing rate normalized to 2 min. (E and H) Firing duration to last action potential. Six to 7 neurons per group from n = 8 to 17 rats. *P < 0.05, **P < 0.01. (I and J) Effect of Dy4 and Dy4 inact on EPSC in lamina I/IIo induced by primary afferent stimulation, n = 11 neurons. (K and L) Effects of Dy4, PS2, and inactive analogs on capsaicin-stimulated SP-IR (K) and CGRP-IR (L) release from segments of mouse spinal cord. n = 6 experiments. ANOVA, Tukey’s test (B); Sidak’s test (D and G); Dunn’s test (E and H).
Fig. 4. NK1R endocytosis, ERK signaling, and nociception in vivo
Effects of intrathecal (i.t.) injections of inhibitors or siRNA. (A and B) Localization of NK1R-IR (A) and pERK-IR (B) in rat spinal neurons 10 min after intraplantar (i.pl.) vehicle or capsaicin (Cap). L, lamina. (A) Arrowheads show cell surface and arrows show endosomal NK1R. (B) Arrows show pERK-IR (green) and red shows NeuN (neuronal marker). (C and D) Quantification of NK1R endocytosis (C) and pERK-expressing neurons (D). **P < 0.01, ***P < 0.001. Neuronal numbers: Veh, 54; capsaicin, 52; Dy4, 28; Dy4 inact, 18; PS2, 22; PS2 inact, 19 (≥6 neurons in sections from n = 3 rats). (E, F, H, to K) Nociception in mice after intrathecal injection of endocytic inhibitors (Dy4, PS2), NK1R antagonist (SR140,333; SR), dynamin-1 siRNA, βARR1/2 siRNA, endothelin-converting enzyme-1 inhibitor (SM-19712; SM), or MEK inhibitor (U0126). von Frey withdrawal responses of capsaicin-injected (E and H to K) or contralateral (F) paw. (G) Rotarod latency. (L) Formalin (form) nocifensive behavior. (M) von Frey withdrawal responses of the CFA-injected paw. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, to control. Student’s t test (C and D); ANOVA, Dunnett’s test (E to M).
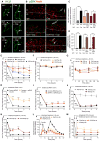
Fig. 5. Disruption of NK1R/βARR interactions
(A) Mouse NK1R C terminus, indicating NK1Rδ311 truncation and sequences of Tat-conjugated NK1R and control peptides. (B and C) SP-induced BRET between WT NK1R-RLUC8 or NK1Rδ311-RLUC8 and βARR2-YFP (B) or RAB5A-Venus (C). Triplicate observations, n ≥ 3 experiments. (D) SP-induced cytosolic ERK (CytoEKAR) and nuclear ERK (NucEKAR) measured using FRET biosensors. *P < 0.05. Forty-nine to 99 cells, three experiments. (E) Effect of SP on SRE-SEAP release from HEK-NK1Rδ311 cells. (F and G) Effect of control and three NK1R peptides on SP-induced NK1R-RLUC8/βARR2-YFP BRET (F) and NK1R endocytosis (G). (H to J) Effects of intrathecally administered control and NK1R peptides on capsaicin-induced mechanical allodynia (H), formalin-evoked nocifensive behavior (I), and CFA-induced mechanical hyperalgesia (J) in mice. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 to control. ANOVA, Sidak’s test (D and G); Dunnett’s test (H to J).
Fig. 6. Antagonism of endosomal NK1R
(A) Structure of tripartite probes. (B) Cy5–ethyl ester or Cy5-Chol uptake in HEK293 cells. (C) Cy5-Chol or Cy5-Span-Chol (red) trafficking to RAB5A–red fluorescent protein (RFP)–positive (blue) and NK1R-GFP–positive (green) endosomes. Asterisk, extracellular; arrowheads, plasma membrane; arrows, endosomes. (D and E) Cy5-Chol:SNAP549-NK1R FRET, indicating localization of SNAP549-NK1R, Cy5-Chol, and FRET signals (D) and time course of FRET in the cytosol (E). Six to nine cells, n = 3 experiments. (F to H) FRET assays of nuclear ERK activity (NucEKAR) immediately after (0 min) (F) or 4 hours after (4 hours) (G) 30 min of preincubation with Span, Span-Chol, or SR140,333 (SR). (H) AUC of (G). **P < 0.01, ***P < 0.001, to vehicle; ^^^P < 0.001, to antagonists. Thirty-one to 417 cells, n = 3 to 5 experiments. (I) Effects of Span or Span-Chol on SP-induced SRE-SEAP. HEK-NK1R cells were pulse-incubated with Span or Span-Chol for 30 min, washed, recovered for 4 hours, and then stimulated with SP for 20 hours (pulse incubation) or were coincubated with antagonists throughout the experiment (coincubation). n = 3 experiments. ANOVA, Sidak’s test (H).
Fig. 7. Antagonism of endosomal NK1R in spinal cord slices and in vivo
(A to C) Effects of tripartite antagonists on SP-induced firing of rat spinal neurons. (A) Representative traces. (B) Firing rate normalized to 2 min. (C) Firing duration to last action potential. Six to seven neurons per group from n = 5 to 18 rats. (D) Localization of Cy5-Chol (arrows, red) and DAP (blue) in superficial laminae (L) 6 hours after intrathecal injection in mouse. Top panel shows phase contrast superimposed on a fluorescence image; bottom panels show fluorescence images. White box denotes magnified region. (E to J) Effects of intrathecally administered Cy5-Chol, SR140,333 (SR), Span, Span-Chol, L-733,066 (L733), or L-733,0660–Chol on nociception in mice. (E to G) von Frey withdrawal responses of capsaicin-injected paw. (H) Nocifensive behavior after intraplantar formalin. (I and J) von Frey withdrawal responses of CFA-injected paw. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, to control. (K) Kinetics of degradation of SP, Span, and Span-Chol by membranes prepared from mouse spinal cord (n = 3). (L) Kinetics of degradation of Span and Span-Chol in human cerebrospinal fluid. n = 2, mean ± SD. ANOVA, Sidak’s test (B); Dunn’s test (C); Dunnett’s test (E to J).
Fig. 8. Endosomal platforms for signaling pain
(A) Nociceptive signaling. NK1R couples to Gαq (1), PLC-dependent Ca2+ mobilization (2), and a disintegrin and metalloproteinase (ADAM)–dependent epidermal growth factor receptor (EGFR) transactivation (3), which stimulates cytosolic ERK (4). Ca2+ activates PKC, which stimulates adenylyl cyclase (AC) to produce plasma membrane cAMP (5). GRK-phosphorylated NK1R interacts with βARRs (6), which scaffold clathrin and adaptor protein 2 (AP2), leading to SP/NK1R endocytosis (7). Endosomal SP/NK1R (8) stimulates cytosolic PKC activity, cytosolic cAMP, and nuclear ERK activity (9), which drive neuronal excitation and nociceptive transmission. (B) Antinociception, endocytic inhibitors. Inhibition of dynamin (1), clathrin (2), or βARRs (3) prevents SP/NK1R endocytosis, endosomal signaling, and nociceptive transmission. (C) Antinociception, tripartite antagonists. Tripartite antagonists incorporate into the plasma membrane (1) and traffic to endosomes (2), where they suppress SP/NK1R nociceptive signaling.
Similar articles
- A lipid-anchored neurokinin 1 receptor antagonist prolongs pain relief by a three-pronged mechanism of action targeting the receptor at the plasma membrane and in endosomes.
Mai QN, Shenoy P, Quach T, Retamal JS, Gondin AB, Yeatman HR, Aurelio L, Conner JW, Poole DP, Canals M, Nowell CJ, Graham B, Davis TP, Briddon SJ, Hill SJ, Porter CJH, Bunnett NW, Halls ML, Veldhuis NA. Mai QN, et al. J Biol Chem. 2021 Jan-Jun;296:100345. doi: 10.1016/j.jbc.2021.100345. Epub 2021 Jan 28. J Biol Chem. 2021. PMID: 33515548 Free PMC article. - Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission.
Yarwood RE, Imlach WL, Lieu T, Veldhuis NA, Jensen DD, Klein Herenbrink C, Aurelio L, Cai Z, Christie MJ, Poole DP, Porter CJH, McLean P, Hicks GA, Geppetti P, Halls ML, Canals M, Bunnett NW. Yarwood RE, et al. Proc Natl Acad Sci U S A. 2017 Nov 14;114(46):12309-12314. doi: 10.1073/pnas.1706656114. Epub 2017 Oct 30. Proc Natl Acad Sci U S A. 2017. PMID: 29087309 Free PMC article. - Therapeutic antagonism of the neurokinin 1 receptor in endosomes provides sustained pain relief.
Hegron A, Peach CJ, Tonello R, Seemann P, Teng S, Latorre R, Huebner H, Weikert D, Rientjes J, Veldhuis NA, Poole DP, Jensen DD, Thomsen ARB, Schmidt BL, Imlach WL, Gmeiner P, Bunnett NW. Hegron A, et al. Proc Natl Acad Sci U S A. 2023 May 30;120(22):e2220979120. doi: 10.1073/pnas.2220979120. Epub 2023 May 22. Proc Natl Acad Sci U S A. 2023. PMID: 37216510 Free PMC article. - Arresting inflammation: contributions of plasma membrane and endosomal signalling to neuropeptide-driven inflammatory disease.
Cattaruzza F, Poole DP, Bunnett NW. Cattaruzza F, et al. Biochem Soc Trans. 2013 Feb 1;41(1):137-43. doi: 10.1042/BST20120343. Biochem Soc Trans. 2013. PMID: 23356273 Review. - Substance P and the Neurokinin-1 Receptor: The New CRF.
Schank JR, Heilig M. Schank JR, et al. Int Rev Neurobiol. 2017;136:151-175. doi: 10.1016/bs.irn.2017.06.008. Epub 2017 Aug 18. Int Rev Neurobiol. 2017. PMID: 29056150 Review.
Cited by
- Signalling of Adrenoceptors: Canonical Pathways and New Paradigms.
Mastos C, Xu X, Keen AC, Halls ML. Mastos C, et al. Handb Exp Pharmacol. 2024;285:147-184. doi: 10.1007/164_2023_704. Handb Exp Pharmacol. 2024. PMID: 38227198 Review. - GPCR kinases differentially modulate biased signaling downstream of CXCR3 depending on their subcellular localization.
Gardner J, Eiger DS, Hicks C, Choi I, Pham U, Chundi A, Namjoshi O, Rajagopal S. Gardner J, et al. Sci Signal. 2024 Feb 13;17(823):eadd9139. doi: 10.1126/scisignal.add9139. Epub 2024 Feb 13. Sci Signal. 2024. PMID: 38349966 Free PMC article. - Endomembrane-Based Signaling by GPCRs and G-Proteins.
Liccardo F, Luini A, Di Martino R. Liccardo F, et al. Cells. 2022 Feb 3;11(3):528. doi: 10.3390/cells11030528. Cells. 2022. PMID: 35159337 Free PMC article. Review. - β-Arrestin-dependent and -independent endosomal G protein activation by the vasopressin type 2 receptor.
Daly C, Guseinov AA, Hahn H, Wright A, Tikhonova IG, Thomsen ARB, Plouffe B. Daly C, et al. Elife. 2023 Oct 19;12:RP87754. doi: 10.7554/eLife.87754. Elife. 2023. PMID: 37855711 Free PMC article. - Targeting endosomal receptors, a new direction for polymers in nanomedicine.
Ramirez-Garcia PD, Veldhuis NA, Bunnett NW, Davis TP. Ramirez-Garcia PD, et al. J Mater Chem B. 2023 Jun 21;11(24):5390-5399. doi: 10.1039/d3tb00156c. J Mater Chem B. 2023. PMID: 37219363 Free PMC article. Review.
References
- Han B, Compton WM, Jones CM, Cai R. Nonmedical prescription opioid use and use disorders among adults aged 18 through 64 years in the United States, 2003–2013. JAMA. 2015;314:1468–1478. - PubMed
- Audet M, Bouvier M. Insights into signaling from the β2-adrenergic receptor structure. Nat Chem Biol. 2008;4:397–403. - PubMed
- Geppetti P, Veldhuis NA, Lieu T, Bunnett NW. G protein-coupled receptors: Dynamic machines for signaling pain and itch. Neuron. 2015;88:635–649. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical