Hornwort Stomata: Architecture and Fate Shared with 400-Million-Year-Old Fossil Plants without Leaves - PubMed (original) (raw)
Hornwort Stomata: Architecture and Fate Shared with 400-Million-Year-Old Fossil Plants without Leaves
Karen S Renzaglia et al. Plant Physiol. 2017 Jun.
Abstract
As one of the earliest plant groups to evolve stomata, hornworts are key to understanding the origin and function of stomata. Hornwort stomata are large and scattered on sporangia that grow from their bases and release spores at their tips. We present data from development and immunocytochemistry that identify a role for hornwort stomata that is correlated with sporangial and spore maturation. We measured guard cells across the genera with stomata to assess developmental changes in size and to analyze any correlation with genome size. Stomata form at the base of the sporophyte in the green region, where they develop differential wall thickenings, form a pore, and die. Guard cells collapse inwardly, increase in surface area, and remain perched over a substomatal cavity and network of intercellular spaces that is initially fluid filled. Following pore formation, the sporophyte dries from the outside inwardly and continues to do so after guard cells die and collapse. Spore tetrads develop in spore mother cell walls within a mucilaginous matrix, both of which progressively dry before sporophyte dehiscence. A lack of correlation between guard cell size and DNA content, lack of arabinans in cell walls, and perpetually open pores are consistent with the inactivity of hornwort stomata. Stomata are expendable in hornworts, as they have been lost twice in derived taxa. Guard cells and epidermal cells of hornworts show striking similarities with the earliest plant fossils. Our findings identify an architecture and fate of stomata in hornworts that is ancient and common to plants without sporophytic leaves.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures
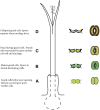
Figure 1.
Diagrammatic representation of a hornwort sporophyte with progressive development and color of stomata indicated from the base upward. Once open, stomata never close, but the outer aperture increases slightly in width after guard cell collapse. The stomata color does not necessarily coincide with the overall color of the sporophyte because stomata die and collapse while the sporophyte is actively photosynthesizing. A, Developing stoma. B, Mature, living, and open stoma. C, Dead (dying) guard cells at the onset of collapse of the outer walls. D, Dead, collapsed, and slightly larger stoma. Above D, the sporophyte dries, leading to dehiscence into two valves along two parallel suture lines, mucilage dries around the spore tetrads, the spore mother cell wall adheres to the spore surfaces, and the spores separate for dispersal.
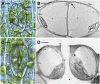
Figure 2.
Phaeoceros carolinianus. A, Differential interference contrast image showing two new guard cells, each with a large amyloplast and an aperture beginning to form in ventral walls (arrow). B, Transmission electron microscopy (TEM) cross section of young guard cells before forming the pore. Each cell contains a large vacuole (v) and plastid (p) with starch. Dorsal (dw), inner (iw), outer (ow), and ventral (vw) walls of the guard cells are thin. The outer ledge (black arrow) and substomatal cavity (white arrow) are beginning to form. C, Differential interference contrast image of older stoma. Each guard cell contains two large amyloplasts, and the aperture (white arrow) is fully developed. Cell walls are thicker than those in A, and epidermal cells contain large amyloplasts (black arrow). D, TEM cross section of a living, fully developed, open stoma with the pore leading to a substomatal cavity (asterisk). Each guard cell contains a thin outer wall (ow), an outer ledge (ol), dorsal (dw) and ventral (vw) walls, and a thickened inner wall (iw). A large vacuole occupies most of the guard cells with nucleus (n) and plastids (p), with pyrenoids (arrow) toward the inside of the stoma. Bars = 10 μm (A and C) and 5 μm (B and D).
Figure 3.
TEM images showing wall ultrastructure in guard cell walls of Leiosporoceros dussii. A, Outer ledge with thickened cuticle (arrow). B, Juncture of inner and ventral guard cell walls with wax deposits on cell walls in the substomatal cavity (arrows). C, Thin fibrillar ventral wall with scattered cuticle/waxes (arrow). D, Thin fibrillar outer wall with a thin layer of cuticle. E, Outer thickened wall with the cuticular layer and cuticle (arrow) of an epidermal cell adjacent to a guard cell. F to J, TEM immunogold localization of pectin epitopes in the guard cell walls of L. dussii. Black dots in images are secondary gold labels attached to specific antibodies. Very strong labeling is shown for LM19 in ventral wall (F), outer wall (G), and inner guard cell wall (H). Scarce labeling is shown for LM6 (I) and LM13 (J), both localized toward the inside of the wall at the plasmalemma. Bars = 0.5 μm except for E, where bar = 2 μm.
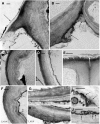
Figure 4.
Stages of senescence and collapse of stomata in three genera of hornworts. The outer aperture remains open and increases in diameter during the drying process. A, P. carolinianus. Scanning electron microscopy (SEM) shows newly opened, slightly raised stoma directly above the involucre. B, L. dussii. Cross section light micrograph of a newly opened stoma shows large starch-filled plastids in guard cells and differentially thickened epidermal and guard cell walls. Small plastids (arrow) occur in epidermal cells, and a substomatal cavity (asterisk) leads to intercellular spaces in the assimilative (cortical) tissue. C, A adscendens Lehm. and Lindenb. SEM cross section shows the epidermis and a stoma with dead collapsing guard cells that contain degenerated protoplasm (arrow). Adjacent epidermal cells have thickened radial walls and are beginning to collapse in the opposite direction from the guard cells. The aperture is wide open superficially, and the thin ventral guard cell walls are buckled. A large substomatal cavity (asterisk) leads to internal air spaces. D, A. adscendens. SEM of stoma shows the onset of guard cell collapse before epidermal cells dry. A thicker cuticle covers epidermal cells compared with guard cells. E, L. dussii. Cross section light micrograph shows dead guard cells with degenerated protoplasm at the onset of collapse of the outer cell wall and while fluid is still within the substomatal cavity (asterisk) and intercellular spaces (double asterisks). Small plastids (arrow) in epidermal cells contrast with large starch-filled plastids (p) in assimilative cells. F, _L. dussii._TEM of dead, collapsed stoma shows the coordinated folding of the thin ventral walls of guard cells. The aperture is open from the outside due to the rigid outer ledges. G, A. adscendens. SEM shows completely collapsed guard cells surrounded by hydrated epidermal cells. Stoma diameter is greater than in the precollapsed guard cell in E. The outer aperture is open, and folded ventral walls of guard cells are visible internally (arrow). H, L. dusii. Cross section light micrograph shows collapsed guard cells. The adjacent epidermal cell contains degenerated cytoplasm and has begun to collapse like an accordion in the opposite direction from the guard cells. Assimilative cells begin to die around the substomatal cavity (asterisk) and intercellular space (double asterisks). I, P. carolinianus. SEM shows the epidermis in desiccated and dehisced sporophyte with ridges of collapsed epidermal cell surrounding an enlarged stoma that has a broadened outer aperture. Bars = 5 μm except for F, where bar = 2 μm.
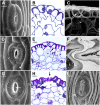
Figure 5.
SEM images of hornwort sporophytes. A to D, P. carolinianus. A, One millimeter of sporogenous tissue extracted from remaining sporophyte shows young tetrads (t) and pseudoelaters (pe) in mucilaginous mass around the columella (c). Stomata collapse at the base where mucilage surrounds tetrads, and through progressive drying of mucilage upwardly, spores and pseudoelaters separate. B, Sporogenous tissue where stomata collapse held together in mucilage (m) showing mature spores of tetrads embedded in the spore mother cell wall (sw) with an imprint of spore wall ornamentation and pseudoelaters. C, Tetrad with spore mother cell wall drying down on the papillate distal wall ornamentation. At this level, stomata are collapsed and internal air spaces are dry within the assimilate tissue. D, Two spores of separated tetrad with a veil of spore mother cell wall adhering to the spore wall. The arrow identifies a spore mother cell wall remnant from a lost spore. E, Large collapsed stoma (arrow) in dried epidermis of a dehiscing Anthoceros cristatus Stephani. sporophyte that contains pseudoelaters separating dried tetrads not surrounded by mucilage or spore mother cell wall. This figure appears courtesy of Silvia Pressel and Jeffrey Duckett. Bars = 100 μm (A), 20 μm (B–D), and = 50 μm (E).
Figure 6.
SEM images of hornwort stomata compared with fossil stomata. A to C, Extant hornwort stomata. A and B, L. dussii. C, P. carolinianus. D to F, Fossil stomata reproduced with permission from Edwards et al. (1998). D, Silurian stoma NMW97.37G.3 with no evidence of two guard cells as in A. The circular pore formed by the outer ledges opens to a constricted aperture below as in B. Epidermal cells are identical to hornwort epidermal cells. E, Early Devonian fossil stoma at the base of terminal sporangium of Sporogonites NMW96.5G.3. Epidermal cells are identical to dried hornwort epidermal cells. F, Silurian stoma NMW94.60G.2 with degenerated outer walls similar to C. Bars = 10 μm.
Figure 7.
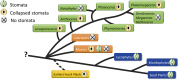
Presence and loss of collapsed stomata in hornworts (green tags). Stomata are plesiomorphic in hornworts, with stomata lost in two clades, Notothylas and the crown group Megaceros/Nothoceros/Dendroceros. The earliest fossil stomata from the Silurian (yellow tag) exhibit the collapsed condition. Among other bryophytes (orange tags), liverworts lack stomata and mosses exhibit all three conditions; Sphagnum has collapsed stomata, and other mosses either possess or have lost stomata. All tracheophytes (blue tags) have green, living stomata. Without a resolution of bryophyte relationships, represented here as a polytomy, it is impossible to determine if stomata are plesiomorphic in embryophytes.
Similar articles
- Hornwort stomata do not respond actively to exogenous and environmental cues.
Pressel S, Renzaglia KS, Dicky Clymo RS, Duckett JG. Pressel S, et al. Ann Bot. 2018 Jun 28;122(1):45-57. doi: 10.1093/aob/mcy045. Ann Bot. 2018. PMID: 29897395 Free PMC article. - Contrasting pectin polymers in guard cell walls of Arabidopsis and the hornwort Phaeoceros reflect physiological differences.
Merced A, Renzaglia KS. Merced A, et al. Ann Bot. 2019 Mar 14;123(4):579-585. doi: 10.1093/aob/mcy168. Ann Bot. 2019. PMID: 30202908 Free PMC article. - Novel insights on the structure and composition of pseudostomata of Sphagnum.
Merced A. Merced A. Am J Bot. 2015 Mar;102(3):329-35. doi: 10.3732/ajb.1400564. Epub 2015 Mar 9. Am J Bot. 2015. PMID: 25784466 - Leaf surface development and the plant fossil record: stomatal patterning in Bennettitales.
Rudall PJ, Bateman RM. Rudall PJ, et al. Biol Rev Camb Philos Soc. 2019 Jun;94(3):1179-1194. doi: 10.1111/brv.12497. Epub 2019 Feb 4. Biol Rev Camb Philos Soc. 2019. PMID: 30714286 Review. - Vegetative and reproductive innovations of early land plants: implications for a unified phylogeny.
Renzaglia KS, Duff RJT, Nickrent DL, Garbary DJ. Renzaglia KS, et al. Philos Trans R Soc Lond B Biol Sci. 2000 Jun 29;355(1398):769-93. doi: 10.1098/rstb.2000.0615. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10905609 Free PMC article. Review.
Cited by
- The hornworts: morphology, evolution and development.
Frangedakis E, Shimamura M, Villarreal JC, Li FW, Tomaselli M, Waller M, Sakakibara K, Renzaglia KS, Szövényi P. Frangedakis E, et al. New Phytol. 2021 Jan;229(2):735-754. doi: 10.1111/nph.16874. Epub 2020 Sep 15. New Phytol. 2021. PMID: 32790880 Free PMC article. Review. - Balancing Strength and Flexibility: How the Synthesis, Organization, and Modification of Guard Cell Walls Govern Stomatal Development and Dynamics.
Rui Y, Chen Y, Kandemir B, Yi H, Wang JZ, Puri VM, Anderson CT. Rui Y, et al. Front Plant Sci. 2018 Aug 20;9:1202. doi: 10.3389/fpls.2018.01202. eCollection 2018. Front Plant Sci. 2018. PMID: 30177940 Free PMC article. Review. - Sporogenesis in Physcomitrium patens: Intergenerational collaboration and the development of the spore wall and aperture.
Renzaglia KS, Ashton NW, Suh DY. Renzaglia KS, et al. Front Cell Dev Biol. 2023 Apr 13;11:1165293. doi: 10.3389/fcell.2023.1165293. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37123413 Free PMC article. - Hornwort stomata do not respond actively to exogenous and environmental cues.
Pressel S, Renzaglia KS, Dicky Clymo RS, Duckett JG. Pressel S, et al. Ann Bot. 2018 Jun 28;122(1):45-57. doi: 10.1093/aob/mcy045. Ann Bot. 2018. PMID: 29897395 Free PMC article. - An Agrobacterium-mediated stable transformation technique for the hornwort model Anthoceros agrestis.
Frangedakis E, Waller M, Nishiyama T, Tsukaya H, Xu X, Yue Y, Tjahjadi M, Gunadi A, Van Eck J, Li FW, Szövényi P, Sakakibara K. Frangedakis E, et al. New Phytol. 2021 Nov;232(3):1488-1505. doi: 10.1111/nph.17524. Epub 2021 Jul 19. New Phytol. 2021. PMID: 34076270 Free PMC article.
References
- Bainard JD, Villarreal JC (2013) Genome size increases in recently diverged hornwort clades. Genome 56: 431–435 - PubMed
- Beaulieu JM, Leitch IJ, Patel S, Pendharkar A, Knight CA (2008) Genome size is a strong predictor of cell size and stomatal density in angiosperms. New Phytol 179: 975–986 - PubMed
- Berry JA, Beerling DJ, Franks PJ (2010) Stomata: key players in the earth system, past and present. Curr Opin Plant Biol 13: 233–240 - PubMed
- Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials