Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia - PubMed (original) (raw)
Clinical Trial
. 2017 Aug 10;130(6):722-731.
doi: 10.1182/blood-2017-04-779405. Epub 2017 Jun 6.
Courtney D DiNardo 3, Daniel A Pollyea 4, Amir T Fathi 5 6, Gail J Roboz 2 7, Jessica K Altman 8, Richard M Stone 9, Daniel J DeAngelo 9, Ross L Levine 1, Ian W Flinn 10, Hagop M Kantarjian 3, Robert Collins 11, Manish R Patel 12, Arthur E Frankel 11, Anthony Stein 13, Mikkael A Sekeres 14, Ronan T Swords 15, Bruno C Medeiros 16, Christophe Willekens 17 18, Paresh Vyas 19 20, Alessandra Tosolini 21, Qiang Xu 21, Robert D Knight 21, Katharine E Yen 22, Sam Agresta 22, Stephane de Botton 17 18, Martin S Tallman 1 2
Affiliations
- PMID: 28588020
- PMCID: PMC5572791
- DOI: 10.1182/blood-2017-04-779405
Clinical Trial
Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia
Eytan M Stein et al. Blood. 2017.
Abstract
Recurrent mutations in isocitrate dehydrogenase 2 (IDH2) occur in ∼12% of patients with acute myeloid leukemia (AML). Mutated IDH2 proteins neomorphically synthesize 2-hydroxyglutarate resulting in DNA and histone hypermethylation, which leads to blocked cellular differentiation. Enasidenib (AG-221/CC-90007) is a first-in-class, oral, selective inhibitor of mutant-IDH2 enzymes. This first-in-human phase 1/2 study assessed the maximum tolerated dose (MTD), pharmacokinetic and pharmacodynamic profiles, safety, and clinical activity of enasidenib in patients with mutant-IDH2 advanced myeloid malignancies. We assessed safety outcomes for all patients and clinical efficacy in the largest patient subgroup, those with relapsed or refractory AML, from the phase 1 dose-escalation and expansion phases of the study. In the dose-escalation phase, an MTD was not reached at doses ranging from 50 to 650 mg per day. Enasidenib 100 mg once daily was selected for the expansion phase on the basis of pharmacokinetic and pharmacodynamic profiles and demonstrated efficacy. Grade 3 to 4 enasidenib-related adverse events included indirect hyperbilirubinemia (12%) and IDH-inhibitor-associated differentiation syndrome (7%). Among patients with relapsed or refractory AML, overall response rate was 40.3%, with a median response duration of 5.8 months. Responses were associated with cellular differentiation and maturation, typically without evidence of aplasia. Median overall survival among relapsed/refractory patients was 9.3 months, and for the 34 patients (19.3%) who attained complete remission, overall survival was 19.7 months. Continuous daily enasidenib treatment was generally well tolerated and induced hematologic responses in patients for whom prior AML therapy had failed. Inducing differentiation of myeloblasts, not cytotoxicity, seems to drive the clinical efficacy of enasidenib. This trial was registered at www.clinicaltrials.gov as #NCT01915498.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: E.M.S. received grants and personal fees from Celgene Corporation and Agios Pharmaceuticals, Inc. C.D.D. received personal fees and clinical research support from Agios Pharmaceuticals, Inc. and clinical research support from Celgene Corporation. D.A.P. served on the advisory board for Pfizer, Karyopharm, Celgene Corporation, and Agios Pharmaceuticals, Inc. and received grants from Agios Pharmaceuticals, Inc. A.T.F. consulted for and received clinical trial support from Celgene Corporation and Seattle Genetics; served on advisory boards for Merck, Juno, Tolero, and Bexalata; and received clinical trial support from Takeda and Exelixis. G.J.R. consulted for Agios Pharmaceuticals, Inc., Celgene Corporation, Amgen, Amphivena, Astex, AstraZeneca, Celator, Genoptix, Janssen, Juno, MEI Pharma, MedImmune, Novartis, Onconova, Pfizer, Roche/Genentech, and Sunesis; and received research support from Cellectis. J.K.A. received personal fees from Syros, Janssen Pharmaceuticals, Novartis, Seattle Genetics, Spectrum, Ariad, Bristol-Myers Squibb, and Celgene Corporation; and funds to institution for trial participation from MethylGene Inc., Boehringer Ingelheim, Astellas, Agios Pharmaceuticals, Bristol-Myers Squibb, CSL Limited, Cyclacel, Epizyme, Genentech, Pfizer, BioLineRX, and Talon Therapeutics. R.M.S. served on an advisory board for Agios Pharmaceuticals, Inc., Novartis, Celgene Corporation, AbbVie, Karyopharm, Arog, Jansen, Celator/Jazz, Seattle Genetics, and Roche/Genetech; and served on data and safety monitoring boards for Celgene Corporation and Sunesis. D.J.D. served on the advisory board for Celgene Corporation. R.L.L. received research support from Celgene Corporation and serves on the supervisory board for Qiagen. I.W.F. received grants from Agios Pharmaceuticals, Inc., Acerta, Beigene, Celgene Corporation, Constellation, Curls, FortySeven, Genentech, Gilead, ImmunoGen, Incyte, Infinity, Janssen, Kite, Merck, Novartis, OncoMed, Pfizer, Portola, Seattle Genetics, Takeda, TG Therapeutics, and Trillium. R.C. received clinical research funding from Agios Pharmaceuticals, Inc. and Celgene Corporation. A.S. consulted for Amgen and Stemline. M.A.S. served on the advisory board for Celgene Corporation. R.T.S. received grants from Takeda and personal fees from Novartis, Agios Pharmaceuticals, Inc., and Celgene Corporation. B.C.M. served on advisory boards for Agios Pharmaceuticals, Inc. and Celgene Corporation. C.W. received grants from Agios Pharmaceuticals, Inc. A.T., Q.X., and R.D.K. are employees and stockholders of Celgene Corporation. K.E.Y. and S.A. are employees of Agios Pharmaceuticals, Inc. S.d.B. received personal fees from Agios Pharmaceuticals, Inc., Celgene Corporation, Novartis, Pfizer and Servier. The remaining authors declare no competing financial interests.
Figures
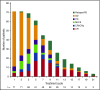
Figure 1.
Evolution of response during treatment of responding patients (n = 71). Bars reflect responses at each cycle. CR, complete response; CRi, CR with incomplete hematologic recovery; CRp, CR with incomplete platelet recovery; MLFS, morphologic leukemia-free state; PD, progressive disease; PR, partial response; SD, stable disease.
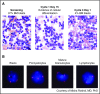
Figure 2.
Morphologic evidence of myeloid differentiation during enasidenib treatment. (A) Bone marrow (BM) blasts at screening (left). By cycle 3 day 1 (right), maturing forms including promyelocytes and myelocytes have largely replaced the immature myeloblasts, without initial marrow aplasia or hypoplasia at cycle 1 day 15 (middle). (B) Fluorescence in situ hybridization evidence of myeloid differentiation during enasidenib treatment. At screening, this patient with an _IDH2_-R140Q mutation had trisomy 8 in the majority of myeloblasts. By cycle 2 day 1, mature forms appeared with persistence of trisomy 8 in promyelocytes and mature granulocytes. In contrast, cells in the lymphoid compartment have a normal complement of chromosome 8.
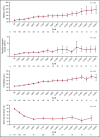
Figure 3.
Mean (± standard error) platelet count, absolute neutrophil count, hemoglobin, and bone marrow blasts over time in patients with relapsed/refractory AML treated with enasidenib.
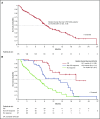
Figure 4.
Overall survival. (A) Overall survival among all patients with relapsed/refractory (R/R) AML. (B) Overall survival among patients with relapsed/refractory AML in complete remission (CR), patients with a non-CR hematologic response, or no response. NE, not evaluated.
Comment in
- Hitting the target in IDH2 mutant AML.
Wouters BJ. Wouters BJ. Blood. 2017 Aug 10;130(6):693-694. doi: 10.1182/blood-2017-06-790394. Blood. 2017. PMID: 28798056 No abstract available.
Similar articles
- Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib.
Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, Roboz GJ, Patel MR, Collins R, Flinn IW, Sekeres MA, Stein AS, Kantarjian HM, Levine RL, Vyas P, MacBeth KJ, Tosolini A, VanOostendorp J, Xu Q, Gupta I, Lila T, Risueno A, Yen KE, Wu B, Attar EC, Tallman MS, de Botton S. Stein EM, et al. Blood. 2019 Feb 14;133(7):676-687. doi: 10.1182/blood-2018-08-869008. Epub 2018 Dec 3. Blood. 2019. PMID: 30510081 Free PMC article. Clinical Trial. - Enasidenib in patients with mutant IDH2 myelodysplastic syndromes: a phase 1 subgroup analysis of the multicentre, AG221-C-001 trial.
Stein EM, Fathi AT, DiNardo CD, Pollyea DA, Roboz GJ, Collins R, Sekeres MA, Stone RM, Attar EC, Frattini MG, Tosolini A, Xu Q, See WL, MacBeth KJ, de Botton S, Tallman MS, Kantarjian HM. Stein EM, et al. Lancet Haematol. 2020 Apr;7(4):e309-e319. doi: 10.1016/S2352-3026(19)30284-4. Epub 2020 Mar 5. Lancet Haematol. 2020. PMID: 32145771 Clinical Trial. - Differentiation Syndrome Associated With Enasidenib, a Selective Inhibitor of Mutant Isocitrate Dehydrogenase 2: Analysis of a Phase 1/2 Study.
Fathi AT, DiNardo CD, Kline I, Kenvin L, Gupta I, Attar EC, Stein EM, de Botton S; AG221-C-001 Study Investigators. Fathi AT, et al. JAMA Oncol. 2018 Aug 1;4(8):1106-1110. doi: 10.1001/jamaoncol.2017.4695. JAMA Oncol. 2018. PMID: 29346478 Free PMC article. Clinical Trial. - Enasidenib: First Mutant IDH2 Inhibitor for the Treatment of Refractory and Relapsed Acute Myeloid Leukemia.
Dogra R, Bhatia R, Shankar R, Bansal P, Rawal RK. Dogra R, et al. Anticancer Agents Med Chem. 2018;18(14):1936-1951. doi: 10.2174/1871520618666181025091128. Anticancer Agents Med Chem. 2018. PMID: 30360730 Review. - An evaluation of enasidenib for the treatment of acute myeloid leukemia.
Del Principe MI, Paterno G, Palmieri R, Maurillo L, Buccisano F, Venditti A. Del Principe MI, et al. Expert Opin Pharmacother. 2019 Nov;20(16):1935-1942. doi: 10.1080/14656566.2019.1654456. Epub 2019 Aug 27. Expert Opin Pharmacother. 2019. PMID: 31454277 Review.
Cited by
- An evaluation of venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine as therapy for acute myeloid leukemia.
Othman TA, Tenold ME, Moskoff BN, Azenkot T, Jonas BA. Othman TA, et al. Expert Rev Hematol. 2021 May;14(5):407-417. doi: 10.1080/17474086.2021.1938533. Epub 2021 Jun 15. Expert Rev Hematol. 2021. PMID: 34076549 Free PMC article. Review. - Digital Droplet PCR is a Specific and Sensitive Tool for Detecting IDH2 Mutations in Acute Myeloid LeuKemia Patients.
Grassi S, Guerrini F, Ciabatti E, Puccetti R, Salehzadeh S, Metelli MR, Di Vita A, Domenichini C, Caracciolo F, Orciuolo E, Pelosini M, Mazzantini E, Rossi P, Mazziotta F, Petrini M, Galimberti S. Grassi S, et al. Cancers (Basel). 2020 Jun 30;12(7):1738. doi: 10.3390/cancers12071738. Cancers (Basel). 2020. PMID: 32629801 Free PMC article. - Has Drug Repurposing Fulfilled its Promise in Acute Myeloid Leukaemia?
Valli D, Gruszka AM, Alcalay M. Valli D, et al. J Clin Med. 2020 Jun 17;9(6):1892. doi: 10.3390/jcm9061892. J Clin Med. 2020. PMID: 32560371 Free PMC article. Review. - Novel Targeted Therapeutics in Acute Myeloid Leukemia: an Embarrassment of Riches.
Grieselhuber NR, Mims AS. Grieselhuber NR, et al. Curr Hematol Malig Rep. 2021 Apr;16(2):192-206. doi: 10.1007/s11899-021-00621-9. Epub 2021 Mar 18. Curr Hematol Malig Rep. 2021. PMID: 33738705 Free PMC article. Review. - Distinguishing AML from MDS: a fixed blast percentage may no longer be optimal.
Estey E, Hasserjian RP, Döhner H. Estey E, et al. Blood. 2022 Jan 20;139(3):323-332. doi: 10.1182/blood.2021011304. Blood. 2022. PMID: 34111285 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- KL2 TR000461/TR/NCATS NIH HHS/United States
- G1000729/MRC_/Medical Research Council/United Kingdom
- MR/L008963/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12009/11/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous