The Role of TRPC6 in the Neuroprotection of Calycosin Against Cerebral Ischemic Injury - PubMed (original) (raw)
The Role of TRPC6 in the Neuroprotection of Calycosin Against Cerebral Ischemic Injury
Chao Guo et al. Sci Rep. 2017.
Abstract
Our previous studies have provided evidences that calycosin can protect the brain from ischemia/reperfusion injury, but its mechanisms is not fully understand. Transient receptor potential canonical 6 (TRPC6) has a critical role in promoting neuronal survival against cerebral ischemic injury. The aim of the present study is to test whether calycosin protects against cerebral ischemic injury through TRPC6-CREB pathway. In vivo, rats were subjected to transient middle cerebral artery occlusion (MCAO) for 2 h and then treated with different doses of calycosin at the onset of reperfusion. In vitro, primary cultured neurons were treated by calycosin, then exposed to 2 h oxygen glucose deprivation (OGD) followed by 24 h reoxygenation. Our results showed that treatment with calycosin protected against ischemia-induced damages by increasing TRPC6 and P-CREB expression and inhibiting calpain activation. The neuroprotection effect of calycosin was diminished by inhibition or knockdown of TRPC6 and CREB. These findings indicated that the potential neuroprotection mechanism of calycosin was involved with TRPC6-CREB pathway.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
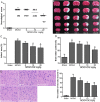
Figure 1
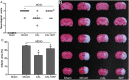
Calycosin (CAL) alleviated MCAO-induced brain injury. (A) Scatterplot of neurological score in the sham, MCAO, 5, 10, 20 mg/kg calycosin treatment groups (Data were presented as median, n = 8). (B) TTC staining of the cerebral infarct in each group. (C) Statistical analysis of the percentage of infarct area in each group (n = 6). (D) The percentage of brain water content in each group (n = 6). (E) H–E stains of coronal sections from the ischemic penumbral cortex in the sham, MCAO, 5, 10, 20 mg/kg calycosin treatment groups. (F) Necrotic neurons were counted in each group (n = 6). All data, except for neurological score, were expressed as mean ± S.D; **P < 0.01 compared with the sham group; ## P < 0.01, # P < 0.05 compared with MCAO group.
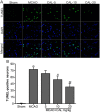
Figure 2
Calycosin (CAL) reduced ischemia-induced apoptosis. (A) Representative images of TUNEL staining of the ischemic penumbral cortex in the sham, MCAO, 5, 10, 20 mg/kg calycosin treatment groups. DAPI stain was used to label the nucleus. (B) Quantitative analysis of the number of TUNEL-positive neurons in each group (n = 6). Data were presented as mean ± S.D. **P < 0.01 compared with the sham group; ## P < 0.01, # P < 0.05 compared with MCAO group.
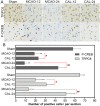
Figure 3
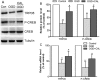
Calycosin (CAL) increased the number of TRPC6 and P-CREB positive cells at 12 and 24 h after reperfusion. (A) Representative sections of immunohistochemical staining in the ischemic penumbral cortex in the sham, MCAO and calycosin treatment groups. (B) Quantitative analysis of the number of positive immune cells (n = 6). Data were expressed as mean ± S.D; **P < 0.01, compared with the sham group; ## P < 0.01, # P < 0.05, compared with MCAO group at the same time point.
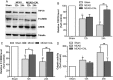
Figure 4
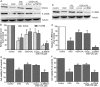
Calycosin (CAL) raised the levels of TRPC6 and P-CREB, and inhibited calpain activation at 12 and 24 h after reperfusion. (A) Representative western blot bands of TRPC6, P-CREB, CREB and tubulin are shown in the sham, MCAO and calycosin treatment groups. (B,C) The quantification analysis of the expression levels of TRPC6 and P-CREB (n = 6). (D) Calpain activities in the sham, MCAO and calycosin treatment groups were measured (n = 6). Data were expressed as mean ± S.D; **P < 0.01, *P < 0.05 compared with the sham group; ## P < 0.01, # P < 0.05, compared with MCAO group at the same time point.
Figure 5
SKF96365 reversed the neuroprotection effect of calycosin. (A) Neurological score in sham, MCAO, calycosin (CAL) and calycosin plus SKF96365 (CAL + SKF) groups (Data were presented as median, n = 6). (B) TTC staining for the cerebral infarct area in each group. (C) Statistical analysis of the percentage of infarct area (n = 6). Data were expressed as mean ± S.D; **P < 0.01, compared with the sham group; # P < 0.05, compared with MCAO group; & P < 0.05, compared with MCAO + CAL group.
Figure 6
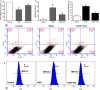
Calycosin (CAL) protected neuron cell against OGD injury. (A) Histogram showing cell viability at 24 h after OGD in control, OGD and OGD + calycosin groups. (B) Representative pictures of flow cytometry analysis for apoptosis in each group; FL1: FITC, FL2: PI. (C) The percentage of apoptotic cells of different groups at 24 h after OGD (n = 6). (D) Measurement of the intracellular Ca2+ concentration by flow cytometry after incubation in fluo-3/AM. (E) The mean fluorescence intensity (MFI) of intracellular Ca2+ in different groups. Data were expressed as mean ± S.D; **P < 0.01, compared with the control group; # P < 0.05, compared with OGD group.
Figure 7
Calycosin (CAL) altered the protein and mRNA levels of TRPC6 and P-CREB at 24 h after OGD. (A) The bands of TRPC6, P-CREB, CREB and tubulin in control, OGD and OGD + calycosin groups. (B) Quantification of TRPC6 and P-CREB levels in each group (n = 6). (C) The mRNA levels were determined by RT-PCR (n = 6). Data were expressed as mean ± S.D; *P < 0.05, **P < 0.01, compared with the corresponding control group (con); # P < 0.05, compared with the corresponding OGD group.
Figure 8
The neuroprotection of calycosin involved the TRPC6/CREB pathway. (A,C) Representative western blot bands showing the expression levels of TRPC6 and P-CREB in each group when cells were transfected with TRPC6 siRNA (si-TRPC6) or CREB siRNA (si-CREB) and control siRNA (si-Con) for 48 h. (B,D) The quantification of TRPC6 and P-CREB levels in each group. (E,F) The percentage of cell viabilities in each group was measured by CCK-8 kit. Data were presented as mean ± S.D. (n = 6). **P < 0.01 compared with the control group; # P < 0.05 compared with OGD group; & P < 0.05 compared with control siRNA (si-Con) group.
Similar articles
- Neuroprotectin D1 attenuates brain damage induced by transient middle cerebral artery occlusion in rats through TRPC6/CREB pathways.
Yao C, Zhang J, Chen F, Lin Y. Yao C, et al. Mol Med Rep. 2013 Aug;8(2):543-50. doi: 10.3892/mmr.2013.1543. Epub 2013 Jun 25. Mol Med Rep. 2013. PMID: 23799606 - Combined bone marrow stromal cells and oxiracetam treatments ameliorates acute cerebral ischemia/reperfusion injury through TRPC6.
Wang J, Sun R, Li Z, Pan Y. Wang J, et al. Acta Biochim Biophys Sin (Shanghai). 2019 Aug 5;51(8):767-777. doi: 10.1093/abbs/gmz059. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 31236585 - Neuroprotective Mechanisms of Calycosin Against Focal Cerebral Ischemia and Reperfusion Injury in Rats.
Wang Y, Ren Q, Zhang X, Lu H, Chen J. Wang Y, et al. Cell Physiol Biochem. 2018;45(2):537-546. doi: 10.1159/000487031. Epub 2018 Jan 25. Cell Physiol Biochem. 2018. PMID: 29402799 - Potential Drug Candidates to Treat TRPC6 Channel Deficiencies in the Pathophysiology of Alzheimer's Disease and Brain Ischemia.
Prikhodko V, Chernyuk D, Sysoev Y, Zernov N, Okovityi S, Popugaeva E. Prikhodko V, et al. Cells. 2020 Oct 24;9(11):2351. doi: 10.3390/cells9112351. Cells. 2020. PMID: 33114455 Free PMC article. Review. - Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke.
Shekhar S, Liu Y, Wang S, Zhang H, Fang X, Zhang J, Fan L, Zheng B, Roman RJ, Wang Z, Fan F, Booz GW. Shekhar S, et al. Int J Mol Sci. 2021 Feb 19;22(4):2074. doi: 10.3390/ijms22042074. Int J Mol Sci. 2021. PMID: 33669830 Free PMC article. Review.
Cited by
- Transient receptor potential ion channels and cerebral stroke.
Xu Q, Zou Y, Miao Z, Jiang L, Zhao X. Xu Q, et al. Brain Behav. 2023 Jan;13(1):e2843. doi: 10.1002/brb3.2843. Epub 2022 Dec 16. Brain Behav. 2023. PMID: 36527242 Free PMC article. Review. - Contribution of TRPC Channels in Neuronal Excitotoxicity Associated With Neurodegenerative Disease and Ischemic Stroke.
Jeon J, Bu F, Sun G, Tian JB, Ting SM, Li J, Aronowski J, Birnbaumer L, Freichel M, Zhu MX. Jeon J, et al. Front Cell Dev Biol. 2021 Jan 8;8:618663. doi: 10.3389/fcell.2020.618663. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33490083 Free PMC article. - Investigation of the Multi-Target Mechanism of Guanxin-Shutong Capsule in Cerebrovascular Diseases: A Systems Pharmacology and Experimental Assessment.
Zhang J, Zhao J, Ma Y, Wang W, Huang S, Guo C, Wang K, Zhang X, Zhang W, Wen A, Shi M, Ding Y. Zhang J, et al. Front Pharmacol. 2021 May 13;12:650770. doi: 10.3389/fphar.2021.650770. eCollection 2021. Front Pharmacol. 2021. PMID: 34054530 Free PMC article. - Regulatory mechanisms of tetramethylpyrazine on central nervous system diseases: A review.
Liu Y, Yang G, Cui W, Zhang Y, Liang X. Liu Y, et al. Front Pharmacol. 2022 Sep 5;13:948600. doi: 10.3389/fphar.2022.948600. eCollection 2022. Front Pharmacol. 2022. PMID: 36133805 Free PMC article. Review. - Interleukin-17 and ischaemic stroke.
Zhang Q, Liao Y, Liu Z, Dai Y, Li Y, Li Y, Tang Y. Zhang Q, et al. Immunology. 2021 Feb;162(2):179-193. doi: 10.1111/imm.13265. Epub 2020 Oct 7. Immunology. 2021. PMID: 32935861 Free PMC article. Review.
References
- Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. Am J Manag Care. 2010;16:525–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources