Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases - PubMed (original) (raw)
Review
. 2017 Jan 23;5(1):e00293.
doi: 10.1002/prp2.293. eCollection 2017 Feb.
Affiliations
- PMID: 28596841
- PMCID: PMC5461643
- DOI: 10.1002/prp2.293
Review
Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases
Md Abdul Alim Al-Bari. Pharmacol Res Perspect. 2017.
Abstract
Emerging viruses such as HIV, dengue, influenza A, SARS coronavirus, Ebola, and other viruses pose a significant threat to human health. Majority of these viruses are responsible for the outbreaks of pathogenic lethal infections. To date, there are no effective therapeutic strategies available for the prophylaxis and treatment of these infections. Chloroquine analogs have been used for decades as the primary and most successful drugs against malaria. Concomitant with the emergence of chloroquine-resistant Plasmodium strains and a subsequent decrease in the use as antimalarial drugs, other applications of the analogs have been investigated. Since the analogs have interesting biochemical properties, these drugs are found to be effective against a wide variety of viral infections. As antiviral action, the analogs have been shown to inhibit acidification of endosome during the events of replication and infection. Moreover, immunomodulatory effects of analogs have been beneficial to patients with severe inflammatory complications of several viral diseases. Interestingly, one of the successful targeting strategies is the inhibition of HIV replication by the analogs in vitro which are being tested in several clinical trials. This review focuses on the potentialities of chloroquine analogs for the treatment of endosomal low pH dependent emerging viral diseases.
Keywords: Chloroquine analogs; antiviral actions; endosomal pH and viral replication.
Figures
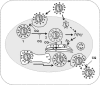
Figure 1
Inhibition of viral infection with the increase
pH
by chloroquine analogs ((Al‐Bari 2015). Steps: 1. Endosome formation; 2. Fusion; 3. posttranslational modification; 4. uncoating virus and CQ, chloroquine.
Similar articles
- Effects of chloroquine on viral infections: an old drug against today's diseases?
Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Savarino A, et al. Lancet Infect Dis. 2003 Nov;3(11):722-7. doi: 10.1016/s1473-3099(03)00806-5. Lancet Infect Dis. 2003. PMID: 14592603 Free PMC article. Review. - Chloroquine could be used for the treatment of filoviral infections and other viral infections that emerge or emerged from viruses requiring an acidic pH for infectivity.
Akpovwa H. Akpovwa H. Cell Biochem Funct. 2016 Jun;34(4):191-6. doi: 10.1002/cbf.3182. Epub 2016 Mar 21. Cell Biochem Funct. 2016. PMID: 27001679 Free PMC article. Review. - The Use of Antimalarial Drugs against Viral Infection.
D'Alessandro S, Scaccabarozzi D, Signorini L, Perego F, Ilboudo DP, Ferrante P, Delbue S. D'Alessandro S, et al. Microorganisms. 2020 Jan 8;8(1):85. doi: 10.3390/microorganisms8010085. Microorganisms. 2020. PMID: 31936284 Free PMC article. Review. - Repurposing of well-known medications as antivirals: hydroxychloroquine and chloroquine - from HIV-1 infection to COVID-19.
Naghipour S, Ghodousi M, Rahsepar S, Elyasi S. Naghipour S, et al. Expert Rev Anti Infect Ther. 2020 Nov;18(11):1119-1133. doi: 10.1080/14787210.2020.1792291. Epub 2020 Jul 13. Expert Rev Anti Infect Ther. 2020. PMID: 32631083 Review.
Cited by
- Identify potent SARS-CoV-2 main protease inhibitors via accelerated free energy perturbation-based virtual screening of existing drugs.
Li Z, Li X, Huang YY, Wu Y, Liu R, Zhou L, Lin Y, Wu D, Zhang L, Liu H, Xu X, Yu K, Zhang Y, Cui J, Zhan CG, Wang X, Luo HB. Li Z, et al. Proc Natl Acad Sci U S A. 2020 Nov 3;117(44):27381-27387. doi: 10.1073/pnas.2010470117. Epub 2020 Oct 13. Proc Natl Acad Sci U S A. 2020. PMID: 33051297 Free PMC article. - Biomedical nanomaterials for immunological applications: ongoing research and clinical trials.
Lenders V, Koutsoumpou X, Sargsian A, Manshian BB. Lenders V, et al. Nanoscale Adv. 2020 Aug 24;2(11):5046-5089. doi: 10.1039/d0na00478b. eCollection 2020 Nov 11. Nanoscale Adv. 2020. PMID: 36132021 Free PMC article. Review. - Potential ocular and systemic COVID-19 prophylaxis approaches for healthcare professionals.
Shetty R, Lalgudi VG, Khamar P, Gupta K, Sethu S, Nair A, Honavar SG, Ghosh A, D'Souza S. Shetty R, et al. Indian J Ophthalmol. 2020 Jul;68(7):1349-1356. doi: 10.4103/ijo.IJO_1589_20. Indian J Ophthalmol. 2020. PMID: 32587162 Free PMC article. Review. - Tackling the cytokine storm in COVID-19, challenges and hopes.
Abdin SM, Elgendy SM, Alyammahi SK, Alhamad DW, Omar HA. Abdin SM, et al. Life Sci. 2020 Sep 15;257:118054. doi: 10.1016/j.lfs.2020.118054. Epub 2020 Jul 11. Life Sci. 2020. PMID: 32663575 Free PMC article. Review. - Can endolysosomal deacidification and inhibition of autophagy prevent severe COVID-19?
Morris G, Athan E, Walder K, Bortolasci CC, O'Neil A, Marx W, Berk M, Carvalho AF, Maes M, Puri BK. Morris G, et al. Life Sci. 2020 Dec 1;262:118541. doi: 10.1016/j.lfs.2020.118541. Epub 2020 Oct 6. Life Sci. 2020. PMID: 33035581 Free PMC article. Review.
References
- Augustijns P, Geusens P, Verbeke N (1992). Chloroquine levels in blood during chronic treatment of patients with rheumatoid arthritis. Eur J Clin Pharmacol 42: 429–433. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous