STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC - PubMed (original) (raw)
STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC
L L Bu et al. J Dent Res. 2017 Aug.
Abstract
Head and neck cancer is one of the most prevalent cancers around the world. Head and neck squamous cell carcinoma (HNSCC) accounts for nearly 90% of head and neck cancer. In recent years, significant advances have been made in immunotherapy for HNSCC. Although some clinical trials targeting immune checkpoints have shown success, the molecular mechanism for regulation of programmed death 1 (PD-1) and its ligand (PD-L1) is partially understood. In an effort to explore the effect of activation of signal transducers and activators of transcriptions (STAT3) on PD-1/PD-L1, the expression and correlation between phosphorylation of STAT3 and PD-1/PD-L1 were determined with immunostaining of human and mouse HNSCC tissue sections. PD-1/PD-L1 overexpression was found to be significantly associated with p-STAT3 in human and mouse HNSCC. Targeting STAT3 by a small molecule effectively inhibited the expression of PD-L1 in the CAL27 cell line. Furthermore, we found that blockade of STAT3 signaling downregulated PD-1/PD-L1 in a Tgfbr1/Pten 2cKO HNSCC mouse model. These findings suggest that STAT3 signaling plays an important role in PD-1/PD-L1 regulation and the antitumor immune response of HNSCC.
Keywords: S3I-201; cytokine; immune checkpoint; immune escape; immunotherapy; mice model.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures
Figure 1.
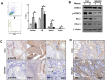
p-STAT3 and PD-1/PD-L1 expression levels in human HNSCC. (A) Representative immunostaining pictures of p-STAT3 (substance), PD-L1, p-STAT3 (mesenchyme), and PD-1 in human HNSCC. Scale bar = 50 µm. (B) Linear regression analysis of p-STAT3 (substance) and PD-L1 histoscore of immunostaining in a human HNSCC tissue microarray; P < 0.001, r = 0.4495. (C) linear regression analysis of p-STAT3 (mesenchyme) and PD-1 histoscore of immunostaining in a human HNSCC tissue microarray; P < 0.001, r = 0.5051. HNSCC, head and neck squamous cell carcinoma; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; STAT, signal transducers and activators of transcription; p-STAT3, phosphorylated STAT3.
Figure 2.
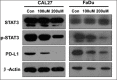
The expression level of PD-L1 was measured by Western blotting in a head and neck squamous cell carcinoma cell line (Cal 27 and FaDu), with and without S3I-201. β-Actin was used as the internal protein loading control. PD-L1, programmed death 1 ligand; STAT, signal transducers and activators of transcription; p-STAT3, phosphorylated STAT3.
Figure 3.
p-STAT3 and PD-1/PD-L1 levels in a Tgfbr1/Pten 2cKO mice model. (A) Representative immunostaining pictures of p-STAT3, PD-L1, and PD-1 in_Tgfbr1/Pten_ 2cKO HNSCC mice. Scale bars = 50 µm. (B) Linear regression analysis of p-STAT3 (substance) and PD-L1 histoscore of immunostaining in Tgfbr1/Pten 2cKO HNSCC mice; P < 0.001, _r_= 0.8041. (C) Linear regression analysis of p-STAT3 (mesenchyme) and PD-1 histoscore of immunostaining in Tgfbr1/Pten 2cKO HNSCC mice; P < 0.001, _r_= 0.7226. HE, hematoxylin and eosin; HNSCC, head and neck squamous cell carcinoma; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; STAT, signal transducers and activators of transcription; p-STAT3, phosphorylated STAT3.
Figure 4.
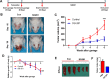
Inhibition of phosphorylation of STAT3 reduced tumor growth in a Tgfbr1/Pten 2cKO mice model. (A) Schematic picture shows the tamoxifen gavage and drug delivery strategy of S3I-201 in Tgfbr1/Pten 2cKO mice. (B) Representative pictures of mice with head and neck tumors after treatment with S3I-201 or PBS on days 18 and 38. (C) Total volume of tumor was measured in the S3I-201 and control groups once a week. Data are presented as mean ± SEM (unpaired Student’s t test;**P < 0.01,***P < 0.001). (D) Relative growth of body (g) was measured in the S3I-201 and control groups once a week. Data are presented as mean ± SEM (unpaired Student’s t test; NS, not significant). (E) Representative spleen pictures of Tgfbr1/Pten 2cKO mice after treatment with S3I-201 or PBS. (F) Spleen index shows that S3I-201 significantly inhibited compensatory growth of spleen in Tgfbr1/Pten 2cKO mice (1-way analysis of variance with post hoc Tukey test; ***P < 0.001). PBS, phosphate-buffered saline; STAT, signal transducers and activators of transcription.
Figure 5.
S3I-201 treatment downregulates the PD-1/PD-L1 signaling axis in Tgfbr1/Pten 2cKO mice. (A) Representative flow cytometry showed decreased staining population of PD-1 in the S3I-201 treatment group and a quantitative PD-1+cell population in spleen, LN, blood, and tumor in the S3I-201 treatment and control groups (1-way analysis of variance with post hoc Tukey test;*P < 0.05). (B) The intratumor levels of STAT3, p-STAT3, PD-1, and PD-L1 were measured by Western blotting in the S3I-201 treatment and control groups. β-Actin was used as the internal protein loading control. (C) Representative immunostaining pictures of p-STAT3, PD-L1, and PD-1 in tumor derive from Tgfbr1/Pten 2cKO mice in the S3I-201 treatment and control groups. Scale bar = 100 µm. (D) The expression level of INF-γ was accessed by immunohistochemistry in Tgfbr1/Pten 2cKO mice after treatment with S3I-201 or PBS. Scale bar = 100 µm. LN, lymph node; PBS, phosphate-buffered saline; PD-1, programmed death 1; PD-L1, programmed death 1 ligand; STAT, signal transducers and activators of transcription; p-STAT3, phosphorylated STAT3.
Similar articles
- The let-7 family of microRNAs suppresses immune evasion in head and neck squamous cell carcinoma by promoting PD-L1 degradation.
Yu D, Liu X, Han G, Liu Y, Zhao X, Wang D, Bian X, Gu T, Wen L. Yu D, et al. Cell Commun Signal. 2019 Dec 27;17(1):173. doi: 10.1186/s12964-019-0490-8. Cell Commun Signal. 2019. PMID: 31881947 Free PMC article. - Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma.
Rebelatto MC, Midha A, Mistry A, Sabalos C, Schechter N, Li X, Jin X, Steele KE, Robbins PB, Blake-Haskins JA, Walker J. Rebelatto MC, et al. Diagn Pathol. 2016 Oct 8;11(1):95. doi: 10.1186/s13000-016-0545-8. Diagn Pathol. 2016. PMID: 27717372 Free PMC article. Clinical Trial. - Cell genomics and immunosuppressive biomarker expression influence PD-L1 immunotherapy treatment responses in HNSCC-a computational study.
Bates AM, Lanzel EA, Qian F, Abbasi T, Vali S, Brogden KA. Bates AM, et al. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017 Aug;124(2):157-164. doi: 10.1016/j.oooo.2017.05.474. Epub 2017 May 25. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017. PMID: 28756882 Free PMC article. - Current studies of immunotherapy in head and neck cancer.
Dogan V, Rieckmann T, Münscher A, Busch CJ. Dogan V, et al. Clin Otolaryngol. 2018 Feb;43(1):13-21. doi: 10.1111/coa.12895. Epub 2017 May 29. Clin Otolaryngol. 2018. PMID: 28464441 Review. - Immunotherapy in head and neck cancers: A new challenge for immunologists, pathologists and clinicians.
Outh-Gauer S, Alt M, Le Tourneau C, Augustin J, Broudin C, Gasne C, Denize T, Mirghani H, Fabre E, Ménard M, Scotte F, Tartour E, Badoual C. Outh-Gauer S, et al. Cancer Treat Rev. 2018 Apr;65:54-64. doi: 10.1016/j.ctrv.2018.02.008. Epub 2018 Mar 1. Cancer Treat Rev. 2018. PMID: 29547766 Review.
Cited by
- STAT3 promotes differentiation of monocytes to MDSCs via CD39/CD73-adenosine signal pathway in oral squamous cell carcinoma.
Cui H, Lan Z, Zou KL, Zhao YY, Yu GT. Cui H, et al. Cancer Immunol Immunother. 2023 May;72(5):1315-1326. doi: 10.1007/s00262-022-03336-9. Epub 2022 Nov 27. Cancer Immunol Immunother. 2023. PMID: 36436019 Free PMC article. - Oral squamous cell carcinomas: state of the field and emerging directions.
Tan Y, Wang Z, Xu M, Li B, Huang Z, Qin S, Nice EC, Tang J, Huang C. Tan Y, et al. Int J Oral Sci. 2023 Sep 22;15(1):44. doi: 10.1038/s41368-023-00249-w. Int J Oral Sci. 2023. PMID: 37736748 Free PMC article. Review. - Role of STAT3 in pancreatic cancer.
Hamel Z, Sanchez S, Standing D, Anant S. Hamel Z, et al. Explor Target Antitumor Ther. 2024;5(5):20-34. doi: 10.37349/etat.2024.00202. Epub 2024 Jan 17. Explor Target Antitumor Ther. 2024. PMID: 38464736 Free PMC article. Review. - Immune subtyping for pancreatic cancer with implication in clinical outcomes and improving immunotherapy.
Liu J, Liu Q, Zhang X, Cui M, Li T, Zhang Y, Liao Q. Liu J, et al. Cancer Cell Int. 2021 Feb 26;21(1):137. doi: 10.1186/s12935-021-01824-z. Cancer Cell Int. 2021. PMID: 33637086 Free PMC article. - Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma.
Song TL, Nairismägi ML, Laurensia Y, Lim JQ, Tan J, Li ZM, Pang WL, Kizhakeyil A, Wijaya GC, Huang DC, Nagarajan S, Chia BK, Cheah D, Liu YH, Zhang F, Rao HL, Tang T, Wong EK, Bei JX, Iqbal J, Grigoropoulos NF, Ng SB, Chng WJ, Teh BT, Tan SY, Verma NK, Fan H, Lim ST, Ong CK. Song TL, et al. Blood. 2018 Sep 13;132(11):1146-1158. doi: 10.1182/blood-2018-01-829424. Epub 2018 Jul 27. Blood. 2018. PMID: 30054295 Free PMC article.
References
- Bellmunt J, Powles T, Vogelzang NJ. 2017. A review on the evolution of PD-1/PD-L1 immunotherapy for bladder cancer: the future is now. Cancer Treat Rev. 54:58–67. - PubMed
- Bromberg J, Darnell JE., Jr. 2000. The role of stats in transcriptional control and their impact on cellular function. Oncogene. 19(21):2468–2473. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous