JNK Activation Turns on LPS- and Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis - PubMed (original) (raw)
JNK Activation Turns on LPS- and Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis
Meraj A Khan et al. Sci Rep. 2017.
Abstract
Neutrophils cast neutrophil extracellular traps (NETs) to ensnare microbial pathogens. Nevertheless, the molecular rheostats that regulate NETosis in response to bacteria are not clearly established. We hypothesized that stress-activated protein kinase or c-Jun N-terminal Kinase (SAPK/JNK) is a molecular switch that turns on NETosis in response to increasing concentrations of lipopolysaccharide (LPS)- and Gram-negative bacteria. Here we show that Escherichia coli LPS (0111:B4; 10-25 μg/ml), but not phorbol myristate acetate (PMA), activates JNK in human neutrophils in a dose-dependent manner. JNK inhibitors SP600125 and TCSJNK6o, and a TLR4 inhibitor TAK242 suppress reactive oxygen species production and NETosis in LPS-, but not PMA-treated neutrophils. Diphenyleneiodonium suppresses LPS-induced NETosis, confirming that endotoxin induces NADPH oxidase-dependent NETosis. Immunoblots, Sytox Green assays, and confocal microscopy of cleaved caspase-3 and nuclear morphology show that JNK inhibition does not induce apoptosis in LPS-stimulated neutrophils. JNK inhibition also suppresses NETosis induced by two typical Gram-negative bacteria, E. coli and Pseudomonas aeruginosa. Therefore, we propose that neutrophils use a TLR4-dependent, JNK-mediated molecular sensing mechanism to initiate NADPH oxidase-dependent suicidal NETosis in response to increasing concentrations of LPS, and Gram-negative bacteria. The LPS-TLR4-JNK activation axis determines the fate of these cells: to be or not to be NETotic neutrophils.
Conflict of interest statement
N.P. filed a patent to regulate NETosis for treating infectious, inflammatory and autoimmune diseases, and was a consultant to Kyowa Hakko Kirin, Co., Ltd.
Figures
Figure 1
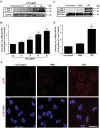
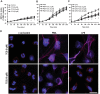
LPS, but not PMA, dose-dependently activates JNK in human neutrophils. (A) Human neutrophils were stimulated with E. coli LPS (0111:B4; 0, 0.1, 1.0, 10, 25 μg/ml) for 30 min, and lyzed for Western blot analyses. Immunoblots show an LPS dose-dependent phosphorylation of JNK (p-JNK). GAPDH blots were used as loading controls (n = 3). (B) The densitometry analyses show the significant dose-dependent increase of the JNK activation in LPS (10 and 25 μg/ml) treated neutrophils. The values were normalized to the negative control values of the same experiment (*indicates p-value < 0.05; One-sample t test compare to hypothetical value 1). (C) Human neutrophils were stimulated with media (-ve control), PMA (25 nM) or LPS (25 μg/ml) for 30 minutes. Immunoblots show that LPS, but not PMA activates JNK in neutrophils. GAPDH blots were used as loading controls (n = 5). (D) The densitometry and statistical analyses were conducted for C, as of B. Total JNK1 and JNK 2 levels do not change within 30-minute incubation period (Supplementary Fig. S1). Error bars in all the panels represent SEM. (E) Confocal microscopy images of the neutrophils immunostained with p-JNK (red), and DNA (blue) after 30 min of NETosis induction, confirm the increased activation of JNK in LPS-, but not PMA-mediated NETosis (n = 3; scale bar 25 μm). See the full Western blots in Supplementary Fig. S1.
Figure 2
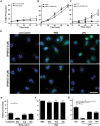
LPS-mediated ROS production in human neutrophils depends on TLR4 signaling and JNK activation. Human neutrophils were treated with cytosolic ROS indicator dye DHR123 and activated with PMA (25 nM), LPS (25 μg/ml) or only media (-ve control) in the presence or absence of JNK inhibitor SP600125 or TCSJNK6o (TCS), or TLR4-TIRAP/TRAM inhibitor TAK242 (TAK). (A–C; E–G) R123 based ROS generation kinetics by plate reader assays show that both PMA and LPS induce ROS generation, over 40 min of post activation. JNK inhibition with both SP600125 and TCSJNK6o drastically reduces the generation of ROS in LPS treated Neutrophils, while inhibitors do not suppress ROS generation in neutrophils activated with PMA. The ROS values were calculated by considering the PMA-mediated ROS production as 100% at 40-minute time point (n = 3, *p-value < 0.05; Two-way ANOVA with Bonferroni’s multiple comparison post test). (D) Confirmation of generation and inhibition of ROS by confocal imaging. R123 (green) and DNA (blue). Confocal images confirm the inhibition of ROS in LPS activated neutrophils but not in neutrophils activated with PMA, at 60-minute time point (n = 3; scale bar 20 μm). (E–G) Measuring R123-based ROS generation kinetics by plate reader assays show that TLR4-TIRAP/TRAM inhibition with TAK242 significantly reduces the generation of ROS in LPS treated Neutrophils. However, the inhibitor does not suppress ROS generation in neutrophils activated with PMA. The ROS values were calculated by considering the PMA-mediated ROS production as 100%, at 40-minute time point (n = 3, *p-value < 0.05; One-way ANOVA with Tukey’s post test compared to negative control). See Supplementary Fig. S2 for relative changes in ROS production. Error bars in all the panels represent SEM.
Figure 3
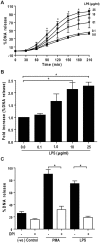
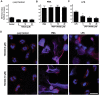
LPS dose-dependently induce Nox-dependent NETosis, and JNK activation is upstream of Nox activation. (A, B) LPS dose (0, 0.1, 1, 10, 25 μg/ml)-dependent NETosis kinetics was assessed by Sytox Green plate reader assays. (A) Considerable NETosis is not detected in the presence of lower concentrations of LPS (e.g., 100 ng/ml). At 1 μg/ml LPS concentration, NETosis is highly variable. Substantial amount of NETosis is detectable in the presence of higher concentrations of (10–25 μg/ml) LPS. %DNA release (NETosis) at the last time point with 25 μg/ml LPS is considered as 100% (n = 3; Two-way ANOVA with Bonferroni’s multiple comparison post test). (B) Fold differences in NETosis was calculated from Panel A (n = 3; One-sample t test compare to hypothetical value 1). (C) Sytox Green plate reader assays show that Nox inhibitor DPI suppresses LPS-mediated NETosis. Inhibition of PMA-mediated NETosis confirms that DPI inhibits Nox-dependent NETosis (n = 3; One-way ANOVA with Tukey’s multiple comparison post test). See Supplementary Fig. S3 for the kinetics of the dose dependent response of DPI during LPS-mediated NETosis. Error bars in all the panels represent SEM.
Figure 4
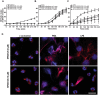
JNK inhibition by SP600125 suppresses LPS-mediated NETosis. (A–C) NETosis kinetics was assessed by Sytox Green plate reader assay after activation with 25 nM PMA and 25 μg/ml LPS in the presence or absence of inhibitor. As shown in the %DNA release analysis, SP600125 (2.5, 5.0, 10 μM) suppresses LPS mediated NETosis in a dosage-dependent manner, while not in PMA mediated NETosis (n = 3–4; *p value < 0.05; Two-way ANOVA with Bonferroni’s post test conducted at each time point). Error bars in all the panels represent SEM. (D) Neutrophils were activated by PMA and LPS with and without SP600125 for 4 hours, immunostained, and imaged for myeloperoxidase (MPO) and DNA. MPO is visible around the nuclei in media control with or without SP600125. MPO co-localizes to NET DNA generated by LPS, PMA, and PMA with SP600125. Neutrophils treated with LPS and SP600125 do not show NETosis, and the nuclear morphology of these cells remains the same as that of the unstimulated control neutrophils (Blue, DAPI staining for DNA; Red, MPO; n = 3; scale bar 20 μm). See Supplementary Fig. S4 for low magnification images.
Figure 5
JNK inhibition by TCSJNK6o suppresses LPS-mediated NETosis. (A–C) NETosis kinetics was assessed by Sytox Green plate reader assay after activation with 25 nM PMA and 25 μg/ml LPS in the presence or absence of TCSJNK6o. As shown in the %DNA release analysis, TCSJNK6o (TCS; 5, 10, 20 μM) suppresses LPS mediated NETosis, while not in PMA mediated NETosis (n = 3–4; *p value < 0.05; Two-way ANOVA with Bonferroni’s post test conducted at each time point). Error bars in all the panels represent SEM. (D) Neutrophils were activated by PMA and LPS with and without TCSJNK6o for 4 hours, immunostained, and imaged for myeloperoxidase (MPO) and DNA. MPO is visible around the nuclei in media control with or without TCSJNK6o. MPO co-localizes to NET DNA generated by LPS, PMA, and PMA with TCSJNK6o. Treating neutrophils with LPS in the presence of TCSJNK6o does not result in NETosis, and the nuclear morphology of these cells remains the same as that of the unstimulated control neutrophils (Blue, DAPI staining for DNA; Red, MPO; n = 3; scale bar 20 μm). See Supplementary Fig. S5 for low magnification images.
Figure 6
TLR4 signaling inhibition by TAK242 suppresses LPS-mediated NETosis. (A–C) NETosis kinetics was assessed by Sytox Green plate reader assay after activation with 25 nM PMA and 25 μg/ml LPS in the presence or absence of TAK242. As shown in the %DNA release analysis, TAK242 (5, 10, 20 μM) suppresses LPS mediated NETosis, while not in PMA mediated NETosis (n = 3–4; *p value < 0.05; One-way ANOVA with Tukey’s multiple comparison post test). Error bars in all the panels represent SEM. (D) Neutrophils were activated by PMA and LPS with and without TAK242 for 4 hours, immunostained, and imaged for myeloperoxidase (MPO) and DNA. MPO is visible around the nuclei in media control with or without TAK242. MPO co-localizes to NET DNA generated by LPS, PMA, and PMA with TAK242. Treating neutrophils with LPS in the presence of TAK242 does not results in NETosis, and the nuclear morphology of these cells remains the same as that of the unstimulated control neutrophils (Blue, DAPI staining for DNA; Red, MPO; n = 3; scale bar 20 μm). See Supplementary Fig. S6 for low magnification images.
Figure 7
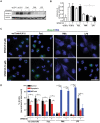
Inhibition of JNK in LPS-treated neutrophils does not lead to apoptosis, and maintains cell survival. (A–B) Immunoblot analyses of the cleave caspase-3 (cCasp-3) show that neutrophils treated with media control for 4 hours in the presence or absence of SP600125 undergo apoptosis to the extent similar to that of Fas-L treatment. Neutrophils activated by 25 nM PMA and 25 μg/ml LPS mostly show NETosis. Treating neutrophils with LPS and SP600125 shows no cCasp-3; NETosis in these cells are very low (*p < 0.05; One-way ANOVA with Tukey’s multiple comparison post test). (C) Confocal microscopy of the neutrophils stained with DAPI (blue) and cCasp-3 (green) after 4 hours. Images show some of the non-activated control neutrophils stain for cCasp-3, in the presence or absence of SP600125. Treating neutrophils with LPS, PMA and PMA with SP600125 exclusively show signs of NETosis; only traces of cCasp-3 is visible in a few of these cells. Treating neutrophils with LPS and SP600125 show no cCasp-3 or NETs (n = 3; scale bar 20 μm). See Supplementary Fig. S7 for the low magnification images. (D) The percentages of normal, apoptotic and NETotic cells were calculated based on cCasp3 staining and nuclear morphology. The quantitative analysis confirms the qualitative analysis (n = 3; *p < 0.05; One-way ANOVA with Tukey’s multiple comparison post test). Error bars in all the panels represent SEM. Collectively, inhibition of JNK activation during LPS-mediated activation of neutrophils result is cell survival.
Figure 8
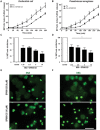
JNK inhibition suppresses both Escherichia coli- and _Pseudomonas aeruginosa-_mediated NETosis. (A-B) Sytox Green assays show the induction of NETosis by both E. coli (EC) and P. aeruginosa (PA; n = 3; *p value < 0.05; t-test). (C–D) Pretreatment of neutrophils with SP600125 and subsequent activation by E. coli and P. aeruginosa (0, 1.25, 2.5, 5.0, 10 MOI each), show significant increase in NETosis inhibition in each MOIs (n = 3; *p value < 0.05; One-way ANOVA with Dunnett’s post test). The NETosis induced by each bacterium in the absence of SP600125 was considered as 100%. Error bars in all the panels represent SEM. (E–F) Confocal images captured on the same day after completing the 4 hour NETosis assays, show clear NETs DNA (green) in E. coli and P. aeruginosa induced neutrophils along with NETosis inhibition in case of SP600125 pretreated conditions (n = 3; scale bar 25 μm).
Figure 9
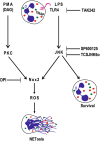
LPS-TLR4-JNK axis regulates Nox-dependent suicidal NETosis. PMA and LPS regulate ROS production in neutrophils via PKC and JNK, respectively. Blocking TLR4 signaling with TAK242 and JNK activation with SP600125 or TCSJNK6o during LPS-mediated NETosis results in the suppression of ROS production and Nox-dependent NETosis, and increased survival of neutrophils. Nox inhibitor DPI suppresses both PMA- and LPS-mediated NETosis. Color dots represent different granular proteins. Granule decorated strings represent DNA and NETs. Specific inhibitors and their points of inhibitions are indicated. Based on all the data obtained in this study, we propose that LPS-TLR4-JNK signaling cascade acts as a sensor of LPS concentrations and bacterial load, and turns on the neutrophil death mode to the suicidal Nox-dependent NETosis.
Similar articles
- Hypertonic Saline Suppresses NADPH Oxidase-Dependent Neutrophil Extracellular Trap Formation and Promotes Apoptosis.
Nadesalingam A, Chen JHK, Farahvash A, Khan MA. Nadesalingam A, et al. Front Immunol. 2018 Mar 8;9:359. doi: 10.3389/fimmu.2018.00359. eCollection 2018. Front Immunol. 2018. PMID: 29593709 Free PMC article. - Short-Term Fever-Range Hyperthermia Accelerates NETosis and Reduces Pro-inflammatory Cytokine Secretion by Human Neutrophils.
Keitelman IA, Sabbione F, Shiromizu CM, Giai C, Fuentes F, Rosso D, Ledo C, Miglio Rodriguez M, Guzman M, Geffner JR, Galletti J, Jancic C, Gómez MI, Trevani AS. Keitelman IA, et al. Front Immunol. 2019 Oct 18;10:2374. doi: 10.3389/fimmu.2019.02374. eCollection 2019. Front Immunol. 2019. PMID: 31681277 Free PMC article. - Histone Deacetylase Inhibitors Dose-Dependently Switch Neutrophil Death from NETosis to Apoptosis.
Hamam HJ, Palaniyar N. Hamam HJ, et al. Biomolecules. 2019 May 11;9(5):184. doi: 10.3390/biom9050184. Biomolecules. 2019. PMID: 31083537 Free PMC article. - A perspective on NETosis in diabetes and cardiometabolic disorders.
Fadini GP, Menegazzo L, Scattolini V, Gintoli M, Albiero M, Avogaro A. Fadini GP, et al. Nutr Metab Cardiovasc Dis. 2016 Jan;26(1):1-8. doi: 10.1016/j.numecd.2015.11.008. Epub 2015 Nov 25. Nutr Metab Cardiovasc Dis. 2016. PMID: 26719220 Review. - NETosis: Molecular Mechanisms, Role in Physiology and Pathology.
Vorobjeva NV, Chernyak BV. Vorobjeva NV, et al. Biochemistry (Mosc). 2020 Oct;85(10):1178-1190. doi: 10.1134/S0006297920100065. Biochemistry (Mosc). 2020. PMID: 33202203 Free PMC article. Review.
Cited by
- TcpC inhibits neutrophil extracellular trap formation by enhancing ubiquitination mediated degradation of peptidylarginine deiminase 4.
Ou Q, Fang JQ, Zhang ZS, Chi Z, Fang J, Xu DY, Lu KZ, Qian MQ, Zhang DY, Guo JP, Gao W, Zhang NR, Pan JP. Ou Q, et al. Nat Commun. 2021 Jun 9;12(1):3481. doi: 10.1038/s41467-021-23881-8. Nat Commun. 2021. PMID: 34108482 Free PMC article. - Early and Late Processes Driving NET Formation, and the Autocrine/Paracrine Role of Endogenous RAGE Ligands.
Tatsiy O, de Carvalho Oliveira V, Mosha HT, McDonald PP. Tatsiy O, et al. Front Immunol. 2021 Sep 20;12:675315. doi: 10.3389/fimmu.2021.675315. eCollection 2021. Front Immunol. 2021. PMID: 34616390 Free PMC article. - Dopamine induces functional extracellular traps in microglia.
Agrawal I, Sharma N, Saxena S, Arvind S, Chakraborty D, Chakraborty DB, Jha D, Ghatak S, Epari S, Gupta T, Jha S. Agrawal I, et al. iScience. 2021 Jan 6;24(1):101968. doi: 10.1016/j.isci.2020.101968. eCollection 2021 Jan 22. iScience. 2021. PMID: 33458617 Free PMC article. - Lung allograft dysbiosis associates with immune response and primary graft dysfunction.
Nelson NC, Wong KK, Mahoney IJ, Malik T, Rudym D, Lesko MB, Qayum S, Lewis TC, Chang SH, Chan JCY, Geraci TC, Li Y, Pamar P, Schnier J, Singh R, Collazo D, Chang M, Kyeremateng Y, McCormick C, Borghi S, Patel S, Darawshy F, Barnett CR, Sulaiman I, Kugler MC, Brosnahan SB, Singh S, Tsay JJ, Wu BG, Pass HI, Angel LF, Segal LN, Natalini JG. Nelson NC, et al. J Heart Lung Transplant. 2025 Mar;44(3):422-434. doi: 10.1016/j.healun.2024.11.006. Epub 2024 Nov 17. J Heart Lung Transplant. 2025. PMID: 39561864 - TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling.
Ciesielska A, Matyjek M, Kwiatkowska K. Ciesielska A, et al. Cell Mol Life Sci. 2021 Feb;78(4):1233-1261. doi: 10.1007/s00018-020-03656-y. Epub 2020 Oct 15. Cell Mol Life Sci. 2021. PMID: 33057840 Free PMC article. Review.
References
- Takei H, Araki A, Watanabe H, Ichinose A, Sendo F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. Journal of leukocyte biology. 1996;59:229–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous