Inhibition of autophagy by berberine enhances the survival of H9C2 myocytes following hypoxia - PubMed (original) (raw)
Inhibition of autophagy by berberine enhances the survival of H9C2 myocytes following hypoxia
Zhuyin Jia et al. Mol Med Rep. 2017 Aug.
Abstract
Hypoxia may induce apoptosis and autophagy to promote cardiomyocyte injury. The present study investigated the effect of berberine, a natural extract of Rhizoma Coptidis, on hypoxia‑induced autophagy and apoptosis in the H9c2 rat myocardial cell line. Expression levels of apoptosis and autophagy markers were upregulated in H9c2 myocytes during hypoxia and cell viability was reduced. However, berberine significantly reduced hypoxia‑induced autophagy in H9c2 myocytes, as demonstrated by the ratio of microtubule‑associated proteins 1A/1B light chain 3 I/II and the expression levels of B‑cell lymphoma 2 (Bcl‑2)/adenovirus E1B 19 kDa protein‑interacting protein 3, and promoted cell viability. In addition, expression levels of the Bcl‑2 anti‑apoptotic protein were significantly downregulated, and expression levels of pro‑apoptotic proteins Bcl‑2‑associated X protein and cleaved caspase‑3 were upregulated during hypoxia injury in cardiac myocytes. This was reversed by treatment with berberine or the autophagy inhibitor 3‑methyladenine, whereas the autophagy agonist rapamycin had the opposite effects, suggesting that berberine reduces myocyte cell death via inhibition of autophagy and apoptosis during hypoxia. In addition, Compound C, a 5' adenosine monophosphate‑activated protein kinase (AMPK) inhibitor, reduced apoptosis and autophagy in hypoxic myocytes, suggesting that the activation of the AMPK signaling pathway may be involved in this process. These findings suggested that berberine protects cells from hypoxia‑induced apoptosis via inhibition of autophagy and suppression of AMPK activation. Therefore, berberine may be a potential therapeutic agent for the treatment of patients with cardiac myocyte injury and ischemia.
Figures
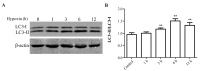
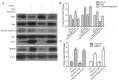
Figure 1.
Expression levels of autophagy-associated proteins following hypoxia in H9c2 myocytes. Following 0–12 h hypoxia, whole cell lysates were collected for western blot analysis. (A) Representative blots of LC3-I, LC3-II and β-actin. (B) Densitometry of LC3-II/LC3-I signals. The protein expression level of the control (0 h) group was arbitrarily set as 1 in each blot, and the target protein signals on the same blot were normalized to the control to generate relative densities. Data are expressed as the mean ± standard deviation and were obtained from three independent experiments. **P<0.01 vs. control. LC3; microtubule-associated proteins 1A/1B light chain 3.
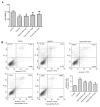
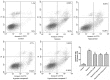
Figure 2.
Effect of berberine on cell viability and apoptosis in H9c2 cells exposed to hypoxia. Cells were treated with 0, 5, 10 or 25 µM berberine, prior to hypoxia. (A) Cell viability of H9c2 cells, as assessed by an MTT assay. (B) Induction of apoptosis in H9c2 cells was measured by Annexin-V/PI double-staining followed by flow cytometric analysis. The upper right and lower right areas, which represented the percentage of late apoptosis and early apoptosis, were analyzed. Data are expressed as the mean ± standard deviation and were obtained from three independent experiments. **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. hypoxia group. Ber, berberine; FITC, fluorescein isothiocyanate; PI, propidium iodide.
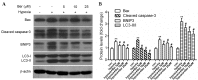
Figure 3.
Berberine inhibits the hypoxia-induced increase in the expression levels of autophagy- and apoptotic-associated proteins. (A) Protein expression levels of Bax, cleaved caspase-3, BNIP3, LC3 and β-actin were measured by western blot analysis. (B) Quantification of bands by densitometry. β-actin served as the loading control. Data are expressed as the mean ± standard deviation and were obtained from three independent experiments. **P<0.01 vs. control group; #P<0.01 vs. hypoxia group. Bax, B-cell lymphoma 2-associated X protein; BNIP3, B-cell lymphoma 2/adenovirus E1B 19 kDa protein-interacting protein 3; LC3, microtubule-associated proteins 1A/1B light chain 3; Ber, berberine.
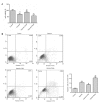
Figure 4.
Effect of 3-MA or rapamycin on cell viability and apoptosis in hypoxic H9c2 cells. Cells were subjected to glucose starvation with the exception of the control group, which was cultured in complete Dulbecco's modified Eagle's medium. Subsequently, cells were treated with 3-MA (an autophagy inhibitor) or rapamycin (an autophagy inducer) prior to hypoxia exposure. (A) Viability of H9c2 cells. (B) Induction of apoptosis in H9c2 cells was measured by Annexin-V/PI double-staining followed by flow cytometric analysis. The apoptotic proportion of H9c2 cells was calculated according to the flow cytometry results. The upper right and lower right areas, which represented the percentage of late apoptosis and early apoptosis, were analyzed. Data are expressed as the mean ± standard deviation and were obtained from three independent experiments. **P<0.01 vs. control group; #P<0.05 and ##P<0.01 vs. hypoxia group. 3-MA, 3-methyladenine; FITC, fluorescein isothiocyanate; PI, propidium iodide; Rapa, rapamycin.
Figure 5.
Inhibition of autophagy reduces apoptosis in hypoxia-induced myocytes. (A) Protein expression levels of Bax, Bcl-2, cleaved caspase-3, BNIP3, LC3 and β-actin were measured by western blot analysis. Quantification of (B) Bcl-2, Bax and cleaved caspase-3, and (C) LC3-II/LC3-I and BNIP3 protein expression levels by densitometry. β-actin served as the loading control. Data are expressed as the mean ± standard deviation and were obtained from three independent experiments. **P<0.01 vs. control group; ##P<0.01 vs. hypoxia group. Bax, B-cell lymphoma 2-associated X protein; Bcl-2, B-cell lymphoma 2; BNIP3, B-cell lymphoma 2/adenovirus E1B 19 kDa protein-interacting protein 3; LC3, microtubule-associated proteins 1A/1B light chain 3; 3-MA, 3-methyladenine.
Figure 6.
Activation of the AMPK signaling pathway may be involved in the regulation of apoptosis by berberine in hypoxic H9c2 cells. Cells were subjected to glucose starvation with the exception of the control group, which was cultured in complete Dulbecco's modified Eagle's medium. Cells were treated with berberine, Compound C (an AMPK inhibitor) or a combination of the two, prior to hypoxia exposure. Induction of apoptosis in H9c2 cells was measured by Annexin-V/PI double-staining followed by flow cytometric analysis. The apoptotic proportion of H9c2 cells was calculated according to the flow cytometry results. The upper right and lower right areas, which represented the percentage of late apoptosis and early apoptosis, were analyzed. Data are expressed as the mean ± standard deviation and were obtained from three independent experiments. **P<0.01 vs. control group; #P<0.05 vs. hypoxia group. AMPK, 5′ adenosine monophosphate-activated protein kinase; FITC, fluorescein isothiocyanate; PI, propidium iodide; Ber, berberine.
Similar articles
- Curcumin inhibits autophagy and apoptosis in hypoxia/reoxygenation-induced myocytes.
Huang Z, Ye B, Dai Z, Wu X, Lu Z, Shan P, Huang W. Huang Z, et al. Mol Med Rep. 2015 Jun;11(6):4678-84. doi: 10.3892/mmr.2015.3322. Epub 2015 Feb 9. Mol Med Rep. 2015. PMID: 25673156 - Lycopene protects against apoptosis in hypoxia/reoxygenation‑induced H9C2 myocardioblast cells through increased autophagy.
Chen F, Sun ZW, Ye LF, Fu GS, Mou Y, Hu SJ. Chen F, et al. Mol Med Rep. 2015 Feb;11(2):1358-65. doi: 10.3892/mmr.2014.2771. Epub 2014 Oct 27. Mol Med Rep. 2015. PMID: 25351505 - Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes.
Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z, Dai K, Wang C, Huang W. Huang Z, et al. Eur J Pharmacol. 2015 Sep 5;762:1-10. doi: 10.1016/j.ejphar.2015.05.028. Epub 2015 May 21. Eur J Pharmacol. 2015. PMID: 26004523 - AMPK and its Activator Berberine in the Treatment of Neurodegenerative Diseases.
Qin S, Tang H, Li W, Gong Y, Li S, Huang J, Fang Y, Yuan W, Liu Y, Wang S, Guo Y, Guo Y, Xu Z. Qin S, et al. Curr Pharm Des. 2020;26(39):5054-5066. doi: 10.2174/1381612826666200523172334. Curr Pharm Des. 2020. PMID: 32445451 Review. - Cardiomyocyte death: mechanisms and translational implications.
Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S. Chiong M, et al. Cell Death Dis. 2011 Dec 22;2(12):e244. doi: 10.1038/cddis.2011.130. Cell Death Dis. 2011. PMID: 22190003 Free PMC article. Review.
Cited by
- Exosomes From Induced Pluripotent Stem Cell-Derived Cardiomyocytes Promote Autophagy for Myocardial Repair.
Santoso MR, Ikeda G, Tada Y, Jung JH, Vaskova E, Sierra RG, Gati C, Goldstone AB, von Bornstaedt D, Shukla P, Wu JC, Wakatsuki S, Woo YJ, Yang PC. Santoso MR, et al. J Am Heart Assoc. 2020 Mar 17;9(6):e014345. doi: 10.1161/JAHA.119.014345. Epub 2020 Mar 5. J Am Heart Assoc. 2020. PMID: 32131688 Free PMC article. - Regulation of autophagy of the heart in ischemia and reperfusion.
Popov SV, Mukhomedzyanov AV, Voronkov NS, Derkachev IA, Boshchenko AA, Fu F, Sufianova GZ, Khlestkina MS, Maslov LN. Popov SV, et al. Apoptosis. 2023 Feb;28(1-2):55-80. doi: 10.1007/s10495-022-01786-1. Epub 2022 Nov 11. Apoptosis. 2023. PMID: 36369366 Review. - Coptidis Rhizoma: a comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology.
Wang J, Wang L, Lou GH, Zeng HR, Hu J, Huang QW, Peng W, Yang XB. Wang J, et al. Pharm Biol. 2019 Dec;57(1):193-225. doi: 10.1080/13880209.2019.1577466. Pharm Biol. 2019. PMID: 30963783 Free PMC article. Review. - Diosmetin alleviates hypoxia‑induced myocardial apoptosis by inducing autophagy through AMPK activation.
Si Q, Shi Y, Huang D, Zhang N. Si Q, et al. Mol Med Rep. 2020 Aug;22(2):1335-1341. doi: 10.3892/mmr.2020.11241. Epub 2020 Jun 16. Mol Med Rep. 2020. PMID: 32627001 Free PMC article. - Berberine modulates cardiovascular diseases as a multitarget-mediated alkaloid with insights into its downstream signals using in silico prospective screening approaches.
Almowallad S, Al-Massabi R. Almowallad S, et al. Saudi J Biol Sci. 2024 May;31(5):103977. doi: 10.1016/j.sjbs.2024.103977. Epub 2024 Mar 11. Saudi J Biol Sci. 2024. PMID: 38510527 Free PMC article.
References
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. - DOI - PubMed
- Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials