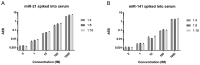Digital direct detection of microRNAs using single molecule arrays - PubMed (original) (raw)
Digital direct detection of microRNAs using single molecule arrays
Limor Cohen et al. Nucleic Acids Res. 2017.
Abstract
MicroRNAs (miRNAs) are involved in many biological pathways, and detecting miRNAs accurately is critical for diagnosing a variety of diseases including cancer. However, most current methods for miRNA detection require lengthy sample preparation and amplification steps that can bias the results. In addition, lack of specificity and reproducibility give rise to various challenges in detection of circulating miRNAs in biological samples. In this work, we applied the Single Molecule Array (Simoa) technique to develop an ultra-sensitive sandwich assay for direct detection of multiple miRNAs without pre-amplification. We successfully detected miRNAs at femtomolar concentrations (with limits of detection [LODs] ranging from 1 to 30 fM) and high specificity (distinguishing miRNAs with a single nucleotide mismatch). This method was effective against a range of diverse target sequences, suggesting a general approach for miRNA detection. To demonstrate the practical application of this technique, we detected miRNAs in a variety of sample types including human serum and total RNA. The high sensitivity and simple workflow of the Simoa method represent excellent advantages for miRNA-based diagnostics of human diseases.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
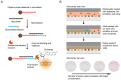
Figure 1.
Schematic of miRNA detection using Simoa. (A) Overview of the sandwich protocol. Capture probes were covalently coupled to microbeads, and then incubated with target miRNA and biotinylated detection probes to form a sandwich complex. The beads were washed and incubated with SβG and RGP (enzyme and substrate) to produce a fluorescent product. (B) Detection was performed in the Simoa format as previously reported (32). As shown in the side view, following hybridization, microbeads suspended in fluorogenic substrate were loaded onto an array of femtoliter-size wells. After loading, the wells were sealed with oil, resulting in an array of isolated reaction chambers each of which contained either zero or one bead. If the enzyme-labeled complex was present on a bead, it generated a fluorescent product resulting in a detectable fluorescent signal. The array was imaged and analyzed to determine the total number of beads, and the number of ‘active’ wells was counted to calculate the average enzyme per bead (AEB). As shown in the top view, the number of active wells increased with increasing target concentration.
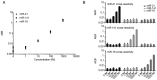
Figure 2.
Calibration curves for various miRNAs. LODs were calculated as three standard deviations above the blank for each assay: (A) miR-16 (LOD 0.76 fM), (B) miR-21 (LOD 1.60 fM), (C) miR-141 (LOD 0.58 fM), (D) miR-25 (LOD 27.34 fM), (E) miR-126 (LOD 8.94 fM) and (F) miR-155 (LOD 4.37 fM).
Figure 3.
Three-plex Simoa assay for miR-21, miR-141 and miR-16, and cross reactivity profiles. (A) Multiplex assay for the simultaneous direct detection of three different miRNAs. (B–D) Cross-reactivity of the multiplex assay in the presence of (B) only miR-21, (C) only miR-141 and (D) only miR-16.
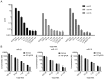
Figure 4.
Three-plex Simoa assay for let-7a, let-7b and let-7c and cross-reactivity profiles. (A) Target, capture, and detection probe sequences used in this assay, and multiplex assay results for the direct detection of three different miRNAs simultaneously. (B–D) Cross-reactivity of the multiplex assay in the presence of (B) only let-7a, (C) only let-7b and (D) only let-7c. (E) Detection of let-7a, let-7b and let-7c in 12 samples using the three-plex Simoa assay. Actual spike-in concentrations for each of let-7a, let-7b and let-7c in the samples (S1–S12) are given in the table, and measured concentrations (after compensating for off-target signal as shown in Supplementary Figure S3 and Supplementary Table S3) are indicated in the plot.
Figure 5.
Direct detection of (A) miR-21 and (B) miR-141 spiked into human serum at varying dilutions.
Figure 6.
Direct detection of miR-21, miR-141 and miR-16 in a total RNA sample. Samples were serially diluted and concentrations were measured using the three-plex Simoa assay. (A) AEB values of the samples at varying amounts of total RNA. (B) Concentrations of miR-21, miR-141 and miR-16 in varying amounts of total RNA measured using RT-qPCR and the three-plex Simoa assay.
Similar articles
- Single molecule measurements of microRNAs in the serum of patients with pulmonary tuberculosis.
Wu Z, Tan Q, Jia X, Wu H, Liang J, Wen W, Wang X, Zhang C, Zhao Y, Chen Y, Luo T, Liu W, Chen X. Wu Z, et al. Front Immunol. 2024 Sep 2;15:1418085. doi: 10.3389/fimmu.2024.1418085. eCollection 2024. Front Immunol. 2024. PMID: 39286248 Free PMC article. - Isothermal Amplification for MicroRNA Detection: From the Test Tube to the Cell.
Deng R, Zhang K, Li J. Deng R, et al. Acc Chem Res. 2017 Apr 18;50(4):1059-1068. doi: 10.1021/acs.accounts.7b00040. Epub 2017 Mar 29. Acc Chem Res. 2017. PMID: 28355077 Review. - A single-molecule method for the quantitation of microRNA gene expression.
Neely LA, Patel S, Garver J, Gallo M, Hackett M, McLaughlin S, Nadel M, Harris J, Gullans S, Rooke J. Neely LA, et al. Nat Methods. 2006 Jan;3(1):41-6. doi: 10.1038/nmeth825. Nat Methods. 2006. PMID: 16369552 - Nanopore single-molecule detection of circulating microRNAs.
Gu LQ, Wang Y. Gu LQ, et al. Methods Mol Biol. 2013;1024:255-68. doi: 10.1007/978-1-62703-453-1_21. Methods Mol Biol. 2013. PMID: 23719958 - Analytical aspects of microRNA in diagnostics: a review.
de Planell-Saguer M, Rodicio MC. de Planell-Saguer M, et al. Anal Chim Acta. 2011 Aug 12;699(2):134-52. doi: 10.1016/j.aca.2011.05.025. Epub 2011 May 20. Anal Chim Acta. 2011. PMID: 21704768 Review.
Cited by
- Single molecule measurements of microRNAs in the serum of patients with pulmonary tuberculosis.
Wu Z, Tan Q, Jia X, Wu H, Liang J, Wen W, Wang X, Zhang C, Zhao Y, Chen Y, Luo T, Liu W, Chen X. Wu Z, et al. Front Immunol. 2024 Sep 2;15:1418085. doi: 10.3389/fimmu.2024.1418085. eCollection 2024. Front Immunol. 2024. PMID: 39286248 Free PMC article. - Multiplexed miRNA and Protein Analysis Using Digital Quantitative PCR in Microwell Arrays.
Vanness BC, Linz TH. Vanness BC, et al. Anal Chem. 2024 Jan 23;96(3):1371-1379. doi: 10.1021/acs.analchem.3c05213. Epub 2024 Jan 6. Anal Chem. 2024. PMID: 38183281 - Digital and Absolute Assays for Low Abundance Molecular Biomarkers.
Kuo CW, Smith AM. Kuo CW, et al. Acc Chem Res. 2023 May 2;56(9):1031-1042. doi: 10.1021/acs.accounts.3c00030. Epub 2023 Apr 17. Acc Chem Res. 2023. PMID: 37068158 Free PMC article. - NF-κB Transcriptional Activity Indispensably Mediates Hypoxia-Reoxygenation Stress-Induced microRNA-210 Expression.
Marwarha G, Slagsvold KH, Høydal MA. Marwarha G, et al. Int J Mol Sci. 2023 Apr 1;24(7):6618. doi: 10.3390/ijms24076618. Int J Mol Sci. 2023. PMID: 37047592 Free PMC article. - Advances in optical counting and imaging of micro/nano single-entity reactors for biomolecular analysis.
Fan W, Ren W, Liu C. Fan W, et al. Anal Bioanal Chem. 2023 Jan;415(1):97-117. doi: 10.1007/s00216-022-04395-8. Epub 2022 Nov 2. Anal Bioanal Chem. 2023. PMID: 36322160 Free PMC article. Review.
References
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297. - PubMed
- Lu J., Getz G., Miska E.A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B.L., Mak R.H., Ferrando A.A. et al. . MicroRNA expression profiles classify human cancers. Nature. 2005; 435:834–838. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources