IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade - PubMed (original) (raw)
Clinical Trial
. 2017 Aug 1;127(8):2930-2940.
doi: 10.1172/JCI91190. Epub 2017 Jun 26.
Jared Lunceford 1, Michael Nebozhyn 1, Erin Murphy 1, Andrey Loboda 1, David R Kaufman 1, Andrew Albright 1, Jonathan D Cheng 1, S Peter Kang 1, Veena Shankaran 2, Sarina A Piha-Paul 3, Jennifer Yearley 1, Tanguy Y Seiwert 4, Antoni Ribas 5, Terrill K McClanahan 1
Affiliations
- PMID: 28650338
- PMCID: PMC5531419
- DOI: 10.1172/JCI91190
Clinical Trial
IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade
Mark Ayers et al. J Clin Invest. 2017.
Abstract
Programmed death-1-directed (PD-1-directed) immune checkpoint blockade results in durable antitumor activity in many advanced malignancies. Recent studies suggest that IFN-γ is a critical driver of programmed death ligand-1 (PD-L1) expression in cancer and host cells, and baseline intratumoral T cell infiltration may improve response likelihood to anti-PD-1 therapies, including pembrolizumab. However, whether quantifying T cell-inflamed microenvironment is a useful pan-tumor determinant of PD-1-directed therapy response has not been rigorously evaluated. Here, we analyzed gene expression profiles (GEPs) using RNA from baseline tumor samples of pembrolizumab-treated patients. We identified immune-related signatures correlating with clinical benefit using a learn-and-confirm paradigm based on data from different clinical studies of pembrolizumab, starting with a small pilot of 19 melanoma patients and eventually defining a pan-tumor T cell-inflamed GEP in 220 patients with 9 cancers. Predictive value was independently confirmed and compared with that of PD-L1 immunohistochemistry in 96 patients with head and neck squamous cell carcinoma. The T cell-inflamed GEP contained IFN-γ-responsive genes related to antigen presentation, chemokine expression, cytotoxic activity, and adaptive immune resistance, and these features were necessary, but not always sufficient, for clinical benefit. The T cell-inflamed GEP has been developed into a clinical-grade assay that is currently being evaluated in ongoing pembrolizumab trials.
Conflict of interest statement
Conflict of interest: M. Ayers, J. Lunceford, M. Nebozhyn, E. Murphy, A. Loboda, D.R. Kaufman, A. Albright, J.D. Cheng, S.P. Kang, J. Yearley, and T.K. McClanahan are employees of Merck & Co. Inc. S.P. Kang and T.K. McClanahan also disclose stock ownership in Merck & Co. Inc. M. Ayers, J. Lunceford, E. Murphy, A. Loboda, and T.K. McClanahan have a patent pending (system and methods for deriving gene signature biomarkers of response to PD-1 antagonists; PCT/US2015/064445), and S.P. Kang has a patent pending (MK-3475). V. Shankaran received a grant from Merck & Co. Inc. during the conduct of the study and has received grants from Amgen and Castle Biosciences. T.Y. Seiwert reports personal fees from Merck & Co. Inc. during the conduct of the study. A. Ribas has been a consultant for Merck & Co. Inc., with the honoraria paid to his institution.
Figures
Figure 1. Gene signature development in melanoma samples.
(A) Overall workflow for the development of immune-related gene signatures that predict response to anti–PD-1 therapy. (B) IFN-γ 10-gene signature evaluated in 19 patients with melanoma and association with response. (C) “Preliminary expanded immune” 28-gene signature with tight correlation to the IFN-γ 10-gene signature, validated in 62 patients with melanoma.
Figure 2. Box plots for the IFN-γ 10-gene and 28-gene expanded immune signatures and best overall response in 62 patients with melanoma with clinical outcomes under anti–PD-1 therapy.
Figure 3. Confirmatory testing and signature refinement across multiple cancer types.
(A and B) Confirmatory analyses of the IFN-γ and expanded immune signature scores for the HNSCC (43 total patients) (A) and gastric cancer (33 patients) (B) cohorts of KEYNOTE-012. (C and D) ROC curves of sensitivity and specificity for the HNSCC (C) and gastric cancer (D) cohorts of KEYNOTE-012.
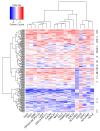
Figure 4. Heatmap for the final 18-gene T cell–inflamed GEP for 216 tumors from patients in KEYNOTE-012 and KEYNOTE-028 considered evaluable for objective response.
Rows represent patients and columns genes. Expression levels have been standardized (centered and scaled) within columns for visualization. The “R” on the right side indicates whether the patient was a responder (by central imaging vendor in KEYNOTE-012 and by investigator assessment in KEYNOTE-028). The rows and columns have been grouped using unsupervised clustering.
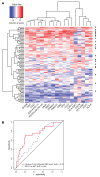
Figure 5. Validation of the final 18-gene T cell–inflamed GEP.
(A) Heatmap of 18-gene T cell–inflamed GEP in 96 PD-L1–unselected patients with HNSCC from KEYNOTE-012. Expression levels have been standardized (centered and scaled) within columns for visualization. The “R” on the right side indicates whether the patient was a responder (by central imaging vendor). The rows and columns have been grouped using unsupervised clustering. (B) ROC curves comparing final 18-gene score with expression of PD-L1 as measured by IHC on tumor and inflammatory cells for a cohort of 96 PD-L1–unselected patients with HNSCC from KEYNOTE-012 considered evaluable for objective response by central imaging vendor.
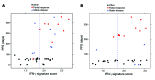
Figure 6. Relationship between increases in IFN-γ immune-related signature score and PFS in response to anti–PD-1 therapy for the HNSCC and gastric cancer cohorts of KEYNOTE-012.
(A) Relationship in the HNSCC cohort (43 total patients). (B) Relationship in the gastric cancer cohort (33 patients). The cutoff associated with the Youden index is displayed in each figure.
Figure 7. PFS time versus T cell–inflamed GEP score in 244 patients from KEYNOTE-012 and KEYNOTE-028 for the 9 cancer cohorts used to determine the T cell–inflamed GEP.
Figure 8. The T cell-inflamed gene expression signature highlights the complex biology of the host immune microenvironment.
Similar articles
- Immune-Related Gene Expression Profiling After PD-1 Blockade in Non-Small Cell Lung Carcinoma, Head and Neck Squamous Cell Carcinoma, and Melanoma.
Prat A, Navarro A, Paré L, Reguart N, Galván P, Pascual T, Martínez A, Nuciforo P, Comerma L, Alos L, Pardo N, Cedrés S, Fan C, Parker JS, Gaba L, Victoria I, Viñolas N, Vivancos A, Arance A, Felip E. Prat A, et al. Cancer Res. 2017 Jul 1;77(13):3540-3550. doi: 10.1158/0008-5472.CAN-16-3556. Epub 2017 May 9. Cancer Res. 2017. PMID: 28487385 - Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer.
Dang TO, Ogunniyi A, Barbee MS, Drilon A. Dang TO, et al. Expert Rev Anticancer Ther. 2016;16(1):13-20. doi: 10.1586/14737140.2016.1123626. Expert Rev Anticancer Ther. 2016. PMID: 26588948 Free PMC article. Review. - Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers.
Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Caliò A, Cuppone F, Sperduti I, Giannarelli D, Chilosi M, Bronte V, Scarpa A, Bria E, Tortora G. Carbognin L, et al. PLoS One. 2015 Jun 18;10(6):e0130142. doi: 10.1371/journal.pone.0130142. eCollection 2015. PLoS One. 2015. PMID: 26086854 Free PMC article. - Predictive biomarkers for programmed death-1/programmed death ligand immune checkpoint inhibitors in nonsmall cell lung cancer.
Remon J, Chaput N, Planchard D. Remon J, et al. Curr Opin Oncol. 2016 Mar;28(2):122-9. doi: 10.1097/CCO.0000000000000263. Curr Opin Oncol. 2016. PMID: 26756384 Review. - T-Cell-Inflamed Gene-Expression Profile, Programmed Death Ligand 1 Expression, and Tumor Mutational Burden Predict Efficacy in Patients Treated With Pembrolizumab Across 20 Cancers: KEYNOTE-028.
Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, Lopez J, Doi T, van Brummelen EMJ, Cristescu R, Yang P, Emancipator K, Stein K, Ayers M, Joe AK, Lunceford JK. Ott PA, et al. J Clin Oncol. 2019 Feb 1;37(4):318-327. doi: 10.1200/JCO.2018.78.2276. Epub 2018 Dec 13. J Clin Oncol. 2019. PMID: 30557521 Clinical Trial.
Cited by
- DDR1 is identified as an immunotherapy target for microsatellite stable colon cancer by CRISPR screening.
Wu M, Ma W, Lv G, Wang X, Li C, Chen X, Peng X, Tang C, Pan Z, Liu R, Chen G, Zhang R. Wu M, et al. NPJ Precis Oncol. 2024 Nov 7;8(1):253. doi: 10.1038/s41698-024-00743-2. NPJ Precis Oncol. 2024. PMID: 39511298 - Predictive biomarkers for immune checkpoint efficacy: is multi-omics breaking the deadlock?
Mogenet A, Greillier L, Tomasini P. Mogenet A, et al. Transl Lung Cancer Res. 2024 Oct 31;13(10):2856-2860. doi: 10.21037/tlcr-24-594. Epub 2024 Oct 17. Transl Lung Cancer Res. 2024. PMID: 39507024 Free PMC article. No abstract available. - Stratifying hepatocellular carcinoma based on immunophenotypes for immunotherapy response and prognosis.
Liu Y, Ji H, Wu LH, Wang XX, Yang Y, Zhang Q, Zhang HM. Liu Y, et al. Mol Ther Oncol. 2024 Oct 5;32(4):200890. doi: 10.1016/j.omton.2024.200890. eCollection 2024 Dec 19. Mol Ther Oncol. 2024. PMID: 39498358 Free PMC article. - Notch1 blockade by a novel, selective anti-Notch1 neutralizing antibody improves immunotherapy efficacy in melanoma by promoting an inflamed TME.
de Freitas JT, Thakur V, LaPorte KM, Thakur VS, Flores B, Caicedo V, Ajaegbu CGE, Ingrasci G, Lipman ZM, Zhang K, Qiu H, Malek TR, Bedogni B. de Freitas JT, et al. J Exp Clin Cancer Res. 2024 Nov 4;43(1):295. doi: 10.1186/s13046-024-03214-5. J Exp Clin Cancer Res. 2024. PMID: 39491031 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials