Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress - PubMed (original) (raw)
Inflammation and vascular remodeling in the ventral hippocampus contributes to vulnerability to stress
J Pearson-Leary et al. Transl Psychiatry. 2017.
Abstract
During exposure to chronic stress, some individuals engage in active coping behaviors that promote resiliency to stress. Other individuals engage in passive coping that is associated with vulnerability to stress and with anxiety and depression. In an effort to identify novel molecular mechanisms that underlie vulnerability or resilience to stress, we used nonbiased analyses of microRNAs in the ventral hippocampus (vHPC) to identify those miRNAs differentially expressed in active (long-latency (LL)/resilient) or passive (short-latency (SL)/vulnerable) rats following chronic social defeat. In the vHPC of active coping rats, miR-455-3p level was increased, while miR-30e-3p level was increased in the vHPC of passive coping rats. Pathway analyses identified inflammatory and vascular remodeling pathways as enriched by genes targeted by these microRNAs. Utilizing several independent markers for blood vessels, inflammatory processes and neural activity in the vHPC, we found that SL/vulnerable rats exhibit increased neural activity, vascular remodeling and inflammatory processes that include both increased blood-brain barrier permeability and increased number of microglia in the vHPC relative to control and resilient rats. To test the relevance of these changes for the development of the vulnerable phenotype, we used pharmacological approaches to determine the contribution of inflammatory processes in mediating vulnerability and resiliency. Administration of the pro-inflammatory cytokine vascular endothelial growth factor-164 increased vulnerability to stress, while the non-steroidal anti-inflammatory drug meloxicam attenuated vulnerability. Collectively, these results show that vulnerability to stress is determined by a re-designed neurovascular unit characterized by increased neural activity, vascular remodeling and pro-inflammatory mechanisms in the vHPC. These results suggest that dampening inflammatory processes by administering anti-inflammatory agents reduces vulnerability to stress. These results have translational relevance as they suggest that administration of anti-inflammatory agents may reduce the impact of stress or trauma in vulnerable individuals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
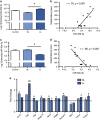
Figure 1
miRNAs and RNAs associated with vascular remodeling increased in vHPC of SL/vulnerable rats. (a) LL/resilient rats exhibited increased levels of miR-455-3p compared with SL/vulnerable rats (_n_=5–7 per group; F2,15=11.49, _P_=0.0009). (b) There was a significant positive correlation between levels of miR-455-3p with average latency to be defeated (_r_=0.79, P<0.001). (c) LL/resilient rats exhibited reduced levels of miR-30e-3p in the vHPC compared with SL/vulnerable rats (_n_=5–7 per group; F2,15=11.12, _P_=0.01). (d) There was a significant negative correlation between levels of miR-30e-3p with average latency to be defeated (_r_=−0.86, P<0.001). For a and c, data represent mean±s.e.m. (e) Angiogenesis array data in SL/vulnerable and LL/resilient rats showing fold change from control indicated by the dashed line (error bars represent s.e.m.). *P<0.05. The letter 'a' denotes statistical significance relative to control and 'b' denotes statistical significance relative to SL. LL, long latency; miRNA, microRNA; SL, short latency; vHPC, ventral hippocampus.
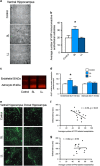
Figure 2
Increases in blood vessel density in rats vulnerable to stress. (a) Representative images of VWF immunohistochemistry. (b) VWF labeling was increased in the vHPC of SL/vulnerable rats relative to LL/resilient rats (_n_=6–7 per group; F2,3=6.02, _P_=0.006). There was no difference in the SL/vulnerable nor LL/resilient rats relative to the controls. (c) Representative western blot images of endothelial GluT1, which was (d) increased in the vHPC of SL/vulnerable rats relative to LL/resilient rats (_n_=6–7 per group; _F_2,17=5.65, _P_=0.013); there was no difference in GluT1 expression between SL/vulnerable nor LL/resilient rats relative to control rats. The astrocytic GluT1 was not altered. (e) Fifty micrometers confocal z-stacks of FITC-labeled blood vessels. (f) There was a negative correlation between latency to social defeat and average number of FITC-labeled vessels in the vHPC (_n_=15, _r_=−0.54, _P_=0.03). (g) There was not a significant correlation between latency to social defeat and the average number of FITC-labeled vessels in the dHPC (_n_=15). Data represent mean+s.e.m. *P<0.05. FITC, fluorescein isothiocyanate; LL, long latency; SL, short latency; vHPC, ventral hippocampus; VWF, von Willebrand Factor.
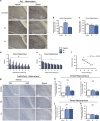
Figure 3
Increases in inflammatory and neural activity in the vHPC of vulnerable rats. (a) Representative immunohistochemistry images for Iba1 showing regions analyzed in bar graphs. (b) Iba1 expression was increased in the vHPC of SL/vulnerable rats relative to control and LL/resilient rats (_n_=7; F2,19=20.78, P<0.0001). (**c**) There was no difference in expression of Iba1 in the dorsal hippocampus between any of the groups (_n_=8 per group). (**d**) Measurement of FITC extravasation showed FITC was significantly increased up to 7 μm away from blood vessels in SL/vulnerable rats relative to control and LL/resilient rats (main effect of group, F2,112=25.83, _P_=0.0001; and main effect of distance, F6,112=4.35, _P_=0.001). (**e**) There were no significant main effect of group between any groups in the dHPC but there was a significant main effect of distance (F6,119=10.76, _P_=0.0001). (**f**) Plasma S100β was negatively correlated with defeat latencies (_n_=11, _r_=−0.75, _P_=0.0009). (**g** and **h**) The long-term neuronal activity marker FosB/ΔFosB was increased in the vHPC CA1 region of SL/vulnerable rats (_n_=5) relative to control (_n_=8) and resilient LL rats (_n_=5; F2,15=18.16, _P_<0.0001). (**i**) There was no difference in FosB/ΔFosB expression in the vHPC CA3 region. (**j** and **k**) There was no difference in FosB/ΔFosB expression between groups in the CA1 (_n_=8 per group) or CA3 region (control _n_=7, SL _n_=8, LL _n_=8) of the dHPC (_P_>0.05). The letter 'a' denotes significant effect of distance and 'b' denotes significant group effect with SL/vulnerable higher than other groups. Data represent mean+s.e.m. *P<0.05. dHPC, dorsal hippocampus; FITC, fluorescein isothiocyanate; LL, long latency; SL, short latency; vHPC, ventral hippocampus.
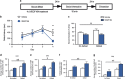
Figure 4
Induction of inflammation in the vHPC promotes stress vulnerability. (a) Experimental design layout. (b) Rats treated with VEGF164 had decreased defeat latencies on day 4 (control _n_=12; VEGF164 _n_=14; _t_24=2.66, _P_=0.01). (c) There was a significant main effect of VEGF on reducing social interaction behavior (_n_=7–8 per group). Subsets of brains were examined for the following measures. (d) There was a main effect of drug treatment (_F_1,13=9.4, _P_=0.009) and social defeat (_F_1,13=21.49, _P_=0.005) on increasing blood vessel density in the CA1 region of the vHPC (_n_=4–5 per group) with VEGF164 significantly increasing density as assessed by VWF and defeated animals having higher VWF expression than non-defeated rats. (e) There was a main effect of drug treatment on VWF expression in the CA3 region of the vHPC (_n_=4–5 per group; _F_1,13=17.2, _P_=0.001) with VEGF164-treated rats exhibiting increased densities. (f) There was a main effect of VEGF164 treatment (_F_1,10=15.01, _P_=0.003) and social defeat (_F_1,10=6.14, _P_=0.03) on Iba1 expression in the ventral hippocampus with increased Iba1 density in both VEGF-treated rats and in defeated rats. (g) There was a main effect of VEGF164 treatment on neural activity (as assessed by counting FosB/ΔFosB immunopositive cells) in the CA1 region of the vHPC (b: _n_=3–4 per group, _F_1,11=8.72, _P_=0.01). There was also a main effect of defeat on neural activity in the CA1 region with defeated rats exhibiting higher neural activity than non-defeated rats (_F_1,11=19.52, _P_=0.001). Data represent mean±s.e.m. and letters denote statistical significance in two-way analysis of variance (ANOVA; P<0.05). The letter 'a' denotes a main effect of defeat, and the letter 'b' denotes a main effect of drug treatment. *P<0.05. vHPC, ventral hippocampus; VWF, von Willebrand Factor.
Figure 5
Inhibition of inflammation in the vHPC reduced indices of vulnerability in SL rats. (a) Experimental design layout. (b) Meloxicam decreased time spent in social interaction compared with vehicle (_n_=5 per group, _t_8=2.5, _P_=0.04), (c) reduced VWF staining (control _n_=4, meloxicam _n_=5, _t_7=2.39, _P_=0.05) (d) and reduced Iba1 staining (_n_=4 per group, _t_6=2.79, _P_=0.03) compared with vehicle treatment of SL rats. (e) Model demonstrating potential mechanism of neural activity and inflammation promoting vascular remodeling in the vHPC during stress and the interaction of these processes leads to vulnerability during stress (passive coping as indicated by shorter latencies to be defeated) and increased anxiety-related behaviors after stress. Data represent mean±s.e.m. *P<0.05. SL, short latency; vHPC, ventral hippocampus; VWF, von Willebrand Factor.
Similar articles
- Interleukin-1α in the ventral hippocampus increases stress vulnerability and inflammation-related processes.
Pearson-Leary J, Eacret D, Bhatnagar S. Pearson-Leary J, et al. Stress. 2020 May;23(3):308-317. doi: 10.1080/10253890.2019.1673360. Epub 2019 Oct 12. Stress. 2020. PMID: 31559913 - Stress primes microglial polarization after global ischemia: Therapeutic potential of progesterone.
Espinosa-Garcia C, Sayeed I, Yousuf S, Atif F, Sergeeva EG, Neigh GN, Stein DG. Espinosa-Garcia C, et al. Brain Behav Immun. 2017 Nov;66:177-192. doi: 10.1016/j.bbi.2017.06.012. Epub 2017 Jun 23. Brain Behav Immun. 2017. PMID: 28648389 - The gut microbiome regulates the increases in depressive-type behaviors and in inflammatory processes in the ventral hippocampus of stress vulnerable rats.
Pearson-Leary J, Zhao C, Bittinger K, Eacret D, Luz S, Vigderman AS, Dayanim G, Bhatnagar S. Pearson-Leary J, et al. Mol Psychiatry. 2020 May;25(5):1068-1079. doi: 10.1038/s41380-019-0380-x. Epub 2019 Mar 4. Mol Psychiatry. 2020. PMID: 30833676 - miRNAs in depression vulnerability and resilience: novel targets for preventive strategies.
Lopizzo N, Zonca V, Cattane N, Pariante CM, Cattaneo A. Lopizzo N, et al. J Neural Transm (Vienna). 2019 Sep;126(9):1241-1258. doi: 10.1007/s00702-019-02048-2. Epub 2019 Jul 26. J Neural Transm (Vienna). 2019. PMID: 31350592 Free PMC article. Review. - Adapting to Stress: Understanding the Neurobiology of Resilience.
Osório C, Probert T, Jones E, Young AH, Robbins I. Osório C, et al. Behav Med. 2017 Oct-Dec;43(4):307-322. doi: 10.1080/08964289.2016.1170661. Epub 2016 Apr 21. Behav Med. 2017. PMID: 27100966 Review.
Cited by
- A New Insight into the Roles of MiRNAs in Metabolic Syndrome.
Huang Y, Yan Y, Xv W, Qian G, Li C, Zou H, Li Y. Huang Y, et al. Biomed Res Int. 2018 Dec 17;2018:7372636. doi: 10.1155/2018/7372636. eCollection 2018. Biomed Res Int. 2018. PMID: 30648107 Free PMC article. Review. - Neurobiology of Resilience: Interface Between Mind and Body.
Cathomas F, Murrough JW, Nestler EJ, Han MH, Russo SJ. Cathomas F, et al. Biol Psychiatry. 2019 Sep 15;86(6):410-420. doi: 10.1016/j.biopsych.2019.04.011. Epub 2019 Apr 17. Biol Psychiatry. 2019. PMID: 31178098 Free PMC article. Review. - CircNFIC Balances Inflammation and Apoptosis by Sponging miR-30e-3p and Regulating DENND1B Expression.
Chen Y, Wang Z, Chen X, Peng X, Nie Q. Chen Y, et al. Genes (Basel). 2021 Nov 19;12(11):1829. doi: 10.3390/genes12111829. Genes (Basel). 2021. PMID: 34828435 Free PMC article. - Blood vessel remodeling in the cerebral cortex induced by binge alcohol intake in mice.
Hasegawa H, Tanaka T, Kondo M, Teramoto K, Nakayama K, Hwang GW. Hasegawa H, et al. Toxicol Res. 2022 Dec 21;39(1):169-177. doi: 10.1007/s43188-022-00164-y. eCollection 2023 Jan. Toxicol Res. 2022. PMID: 36726835 Free PMC article. - The contribution of orexins to sex differences in the stress response.
Grafe LA, Bhatnagar S. Grafe LA, et al. Brain Res. 2020 Mar 15;1731:145893. doi: 10.1016/j.brainres.2018.07.026. Epub 2018 Aug 3. Brain Res. 2020. PMID: 30081036 Free PMC article. Review.
References
- Hammen C. Stress and depression. Annu Rev Clin Psychol 2005; 1: 293–319. - PubMed
- van Praag HM. Can stress cause depression? Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 891–907. - PubMed
- Bowen MT, Dass SA, Booth J, Suraev A, Vyas A, McGregor IS. Active coping toward predatory stress is associated with lower corticosterone and progesterone plasma levels and decreased methylation in the medial amygdala vasopressin system. Horm Behav 2014; 66: 561–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical