Inflammation - Cause or Consequence of Heart Failure or Both? - PubMed (original) (raw)
Review
Inflammation - Cause or Consequence of Heart Failure or Both?
Sophie Van Linthout et al. Curr Heart Fail Rep. 2017 Aug.
Abstract
Purpose of review: With the intention to summarize the currently available evidence on the pathophysiological relevance of inflammation in heart failure, this review addresses the question whether inflammation is a cause or consequence of heart failure, or both.
Recent findings: This review discusses the diversity (sterile, para-inflammation, chronic inflammation) and sources of inflammation and gives an overview of how inflammation (local versus systemic) can trigger heart failure. On the other hand, the review is outlined how heart failure-associated wall stress and signals released by stressed, malfunctioning, or dead cells (DAMPs: e.g., mitochondrial DNA, ATP, S100A8, matricellular proteins) induce cardiac sterile inflammation and how heart failure provokes inflammation in various peripheral tissues in a direct (inflammatory) and indirect (hemodynamic) manner. The crosstalk between the heart and peripheral organs (bone marrow, spleen, gut, adipose tissue) is outlined and the importance of neurohormonal mechanisms including the renin angiotensin aldosteron system and the ß-adrenergic nervous system in inflammation and heart failure is discussed. Inflammation and heart failure are strongly interconnected and mutually reinforce each other. This indicates the difficulty to counteract inflammation and heart failure once this chronic vicious circle has started and points out the need to control the inflammatory process at an early stage avoiding chronic inflammation and heart failure. The diversity of inflammation further addresses the need for a tailored characterization of inflammation enabling differentiation of inflammation and subsequent target-specific strategies. It is expected that the characterization of the systemic and/or cardiac immune profile will be part of precision medicine in the future of cardiology.
Keywords: Cardiosplenic axis; Heart failure; Monocytopoiesis; Para-inflammation; Sterile inflammation; ß-adrenergic signaling.
Conflict of interest statement
Conflict of Interest
Sophie Van Linthout and Carsten Tschöpe declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures
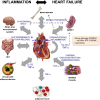
Fig. 1
Inflammation and heart failure reciprocally trigger each other. Heart failure (HF) provokes sterile inflammation in the heart itself triggered by wall stress and signals released by stressed, malfunctioning, or dead cells secondary to HF (DAMPs: e.g., mitochondrial (mt) DNA, ATP, matricellular proteins). The released cardiac cytokines and other inflammatory mediators not only affect the heart but also different organs. IL-1ß induces monocytopoiesis via increasing hematopoietic stem cell proliferation in the bone marrow and monocyte proliferation in the spleen. Cytokines particularly, TNF-α, unleashes inflammation in the skeletal muscle and adipose tissue and accelerate atherogenesis. Furthermore, several neurohormonal mechanisms (renin angiotensin aldosteron system (RAAS) and ß-adrenergic nervous system) that become activated in HF to try and sustain cardiac output in the face of decompensating function also affect inflammation in different organs. ß3 agonism and Ang II induce monocytopoiesis in the spleen. As a consequence of chronic vasoconstriction and underperfusion, inflammation is induced in the skeletal muscle. HF-associated decreased cardiac output and redistribution of systemic circulation can further also lead to a decrease in intestinal perfusion and mucosal ischemia and ultimately, a disrupted intestinal mucosa. This disruption can in turn lead to increased gut permeability and subsequent enhanced translocation of bacteria and bacterial toxins in the blood, which can contribute to systemic inflammation. Systemic inflammation, high-grade (e.g., rheumatoid arthritis) and low-grade (e.g., obesity), and cardiac inflammation induce HF involving different pathophysiological mechanisms. Inflammation triggers cardiomyocyte apoptosis, hypertrophy, stiffness, myofibroblast differentiation, collagen production, endothelial dysfunction, endothelial-to-mesenchymal transition, and subsequent cardiac remodeling and left ventricular dysfunction
Similar articles
- Inflammation and chronic heart failure: from biomarkers to novel anti-inflammatory therapeutic strategies.
Bouras G, Giannopoulos G, Hatzis G, Alexopoulos D, Leventopoulos G, Deftereos S. Bouras G, et al. Med Chem. 2014;10(7):682-99. doi: 10.2174/1573406410666140318113325. Med Chem. 2014. PMID: 25102199 Review. - Translation of hemodynamic stress to sterile inflammation in the heart.
Nakayama H, Otsu K. Nakayama H, et al. Trends Endocrinol Metab. 2013 Nov;24(11):546-53. doi: 10.1016/j.tem.2013.06.004. Epub 2013 Jul 10. Trends Endocrinol Metab. 2013. PMID: 23850260 Review. - [Neurohormonal activity in heart insufficiency].
Svanegaard J, Johansen JB, Thayssen P, Haghfelt T. Svanegaard J, et al. Ugeskr Laeger. 1993 Jun 7;155(23):1784-8. Ugeskr Laeger. 1993. PMID: 8317027 Danish. - The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications.
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. Triposkiadis F, et al. J Am Coll Cardiol. 2009 Nov 3;54(19):1747-62. doi: 10.1016/j.jacc.2009.05.015. J Am Coll Cardiol. 2009. PMID: 19874988 Review. - Nutrition in heart failure: an update.
Sandek A, Doehner W, Anker SD, von Haehling S. Sandek A, et al. Curr Opin Clin Nutr Metab Care. 2009 Jul;12(4):384-91. doi: 10.1097/MCO.0b013e32832cdb0f. Curr Opin Clin Nutr Metab Care. 2009. PMID: 19474718 Review.
Cited by
- Sodium-glucose cotransporter 2 inhibitors' mechanisms of action in heart failure.
Grubić Rotkvić P, Cigrovski Berković M, Bulj N, Rotkvić L, Ćelap I. Grubić Rotkvić P, et al. World J Diabetes. 2020 Jul 15;11(7):269-279. doi: 10.4239/wjd.v11.i7.269. World J Diabetes. 2020. PMID: 32843930 Free PMC article. Review. - Systemic mapping of organ plasma extravasation at multiple stages of chronic heart failure.
Kitzerow O, Suder P, Shukry M, Lisco SJ, Zucker IH, Wang HJ. Kitzerow O, et al. Front Physiol. 2023 Nov 16;14:1288907. doi: 10.3389/fphys.2023.1288907. eCollection 2023. Front Physiol. 2023. PMID: 38033338 Free PMC article. - Effect of azilsartan on myocardial remodeling after acute myocardial infarction.
Wang J, Ding Y, Yao YR, Liu HY, Gu Y. Wang J, et al. Eur J Clin Pharmacol. 2024 Feb;80(2):223-230. doi: 10.1007/s00228-023-03595-0. Epub 2023 Nov 22. Eur J Clin Pharmacol. 2024. PMID: 37991525 Clinical Trial. - Rationale and design of interleukin-1 blockade in recently decompensated heart failure (REDHART2): a randomized, double blind, placebo controlled, single center, phase 2 study.
Van Tassell B, Mihalick V, Thomas G, Marawan A, Talasaz AH, Lu J, Kang L, Ladd A, Damonte JI, Dixon DL, Markley R, Turlington J, Federmann E, Del Buono MG, Biondi-Zoccai G, Canada JM, Arena R, Abbate A. Van Tassell B, et al. J Transl Med. 2022 Jun 15;20(1):270. doi: 10.1186/s12967-022-03466-9. J Transl Med. 2022. PMID: 35706006 Free PMC article. Clinical Trial. - Sex Differences in the Association Between Inflammation and Event-Free Survival in Patients With Heart Failure.
Saleh ZT, Alraoush AT, Aqel AA, Shawashi TO, Chung M, Lennie TA. Saleh ZT, et al. J Cardiovasc Nurs. 2022 Jul-Aug 01;37(4):386-393. doi: 10.1097/JCN.0000000000000831. Epub 2021 Jul 8. J Cardiovasc Nurs. 2022. PMID: 37707972 Free PMC article.
References
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al., M. Authors/Task Force, R. Document. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;2016(18):891–975. - PubMed
- Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials