Modeling the receptor pharmacology, pharmacokinetics, and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy - PubMed (original) (raw)
Modeling the receptor pharmacology, pharmacokinetics, and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy
Deborah Charych et al. PLoS One. 2017.
Abstract
Cytokines are potent immune modulating agents but are not ideal medicines in their natural form due to their short half-life and pleiotropic systemic effects. NKTR-214 is a clinical-stage biologic that comprises interleukin-2 (IL2) protein bound by multiple releasable polyethylene glycol (PEG) chains. In this highly PEG-bound form, the IL2 is inactive; therefore, NKTR-214 is a biologic prodrug. When administered in vivo, the PEG chains slowly release, creating a cascade of increasingly active IL2 protein conjugates bound by fewer PEG chains. The 1-PEG-IL2 and 2-PEG-IL2 species derived from NKTR-214 are the most active conjugated-IL2 species. Free-IL2 protein is undetectable in vivo as it is eliminated faster than formed. The PEG chains on NKTR-214 are located at the region of IL2 that contacts the alpha (α) subunit of the heterotrimeric IL2 receptor complex, IL2Rαβγ, reducing its ability to bind and activate the heterotrimer. The IL2Rαβγ complex is constitutively expressed on regulatory T cells (Tregs). Therefore, without the use of mutations, PEGylation reduces the affinity for IL2Rαβγ to a greater extent than for IL2Rβγ, the receptor complex predominant on CD8 T cells. NKTR-214 treatment in vivo favors activation of CD8 T cells over Tregs in the tumor microenvironment to provide anti-tumor efficacy in multiple syngeneic models. Mechanistic modeling based on in vitro and in vivo kinetic data provides insight into the mechanism of NKTR-214 pharmacology. The model reveals that conjugated-IL2 protein derived from NKTR-214 occupy IL-2Rβγ to a greater extent compared to free-IL2 protein. The model accurately describes the sustained in vivo signaling observed after a single dose of NKTR-214 and explains how the properties of NKTR-214 impart a unique kinetically-controlled immunological mechanism of action.
Conflict of interest statement
Competing Interests: All the authors are employees, receive paid salary, and may own stock options in Nektar Therapeutics. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
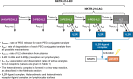
Fig 1. NKTR-214 delivers a controlled, sustained, and biased signal through the IL2 receptor pathway.
NKTR-214 is a CD122-biased cytokine agonist conjugated with multiple releasable chains of PEG located at the interface of IL2 and IL2Rαβγ. The PEG chains slowly release at physiological pH, creating conjugated-IL2 species with fewer PEG chains and increased bioactivity. Sustained signaling through the heterodimeric IL2 receptor pathway (IL2Rβγ) preferentially activates and expands effector CD8 T and NK cells over Tregs.
Fig 2. Parameterization of the mathematical model for NKTR-214 dynamics to simulate concentration-time profiles of conjugated-IL2 species derived from NKTR-214 and to describe receptor occupancy of the conjugated-IL2 species at the IL2Rαβγ and IL2Rβγ.
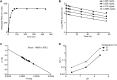
Fig 3. Release of PEG chains follows first-order kinetics.
A. Free PEG detected at various time points during incubation in 0.5 M sodium phosphate buffer at pH 7.4 and 37°C (circles) fit to first-order kinetic profile (solid line), B. Semi-log plot of non-released PEG in phosphate buffer at pH 7.4 starting from indicated concentrations of NKTR-214 at pH 7.4 and 37°C. C. Arrhenius plot of Ln(K) versus 1/Temp is linear. Calculated Ea = 130 kJ/mole for PEG release, D. Effect of pH and temperature on PEG release rate–an equal volume of 0.5 M sodium phosphate buffer at predetermined pH was added to conjugate to produce the final pH of 6.4 or 7.4.
Fig 4. The effect of PEGylation is greatest at the alpha-containing IL2 receptors.
Overlay of RU signals normalized by percentage of IL2 response. Each overlay depicts relative response of 1-PEG-IL2 and 2-PEG-IL2 at A. IL2Rα, B. IL2Rαβ, and C. IL2Rβ. Representative data are shown for each analyte at a concentration that is near the respective IL2 Kd concentration per Table 1.
Fig 5. A single dose of NKTR-214 leads to sustained exposure.
A. Semi-logarithmic plot of plasma concentration vs. time curves for NKTR-214-RC, NKTR-214-AC, and 1-PEG-IL2 after administration of NKTR-214 B. Semi-logarithmic plot of plasma concentration of IL2 after administration of aldesleukin.
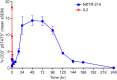
Fig 6. NKTR-214 delivers a controlled and sustained activation signal to the IL2 pathway in vivo measured by pSTAT5 levels in whole peripheral blood.
Mice were treated with a single 0.8 mg/kg dose of either aldesleukin (red) or NKTR-214 (blue) and monitored for up to 240 hours. Peripheral blood was collected at the indicated time points post-dose and pSTAT5 in lymphocyte populations was determined by flow cytometry using antibodies to intracellular pSTAT5 and to extracellular surface marker CD3. N = 5 mice per time point per group.
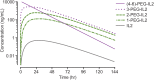
Fig 7. Model-calculated concentration–time profile for species of conjugated-IL2 or free-IL2 that could be theoretically generated from a single dose of 0.8 mg/kg NKTR-214.
Where data are available, the model agrees closely with the PK data shown in Table 3. The model calculates that administration of NKTR-214 results in sustained concentrations of conjugated-IL2 with exceedingly low concentrations of free-IL2, consistent with its fast in vivo clearance and slow formation from NKTR-214.
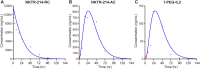
Fig 8. The model suitably fits to measured NKTR-214 concentration-time profiles.
Summing the concentration-time profile of the individual model-derived conjugated-IL2 fit well to the experimentally determined concentration-time profiles of A. NKTR-214-RC, B. NKTR-214-AC, and C. 1-PEG-IL2, the most active conjugate derived from NKTR-214. Solid lines represent the simulation and individual red symbols represent the experimental data.
Fig 9. Conjugated-IL2 species derived from NKTR-214 preferentially occupy IL2Rβγ over IL2Rαβγ.
Percent receptor occupancy calculated by the model from A. 2-PEG-IL2, B. 1-PEG-IL2, and C. free-IL2 derived from NKTR-214, at IL2Rβγ (gray lines) and IL2Rαβγ (green lines) receptor complexes.
Fig 10. Receptor bias is an intrinsic property of NKTR-214.
Simulation of the receptor occupancy percentage over time for therapeutic levels (0.8 mg/kg) of aldesleukin qdx5 (red) or NKTR-214, qd (blue) compared head to head. A. The receptor occupancy area under the curve for IL2Rβγ is 20.8-fold higher after NKTR-214 compared to aldesleukin. B. In contrast, the AUC of IL2Rαβγ is 0.56-fold after NKTR-214 compared to aldesleukin.
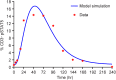
Fig 11. The model correctly estimates experimental % pSTAT5 activation from the receptor occupancy determination.
Model fit (blue line) of pSTAT5 signaling from NKTR-214 as compared to the observed measured values (symbols).
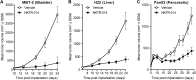
Fig 12. NKTR-214 is consistently effective across several established mouse tumor models.
Examples shown below include A. bladder (MBT-2), B. liver (H22), and C. pancreatic (Pan02) for single-agent NKTR-214 compared to vehicle. In all cases, tumors were grown to large size, 80-100mm3, N = 7/group; NKTR-214 0.8 mg/kg q9dx3.*, P < 0.05 (unpaired t-test).
Similar articles
- NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models.
Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, Sheng D, Liu X, Sims PW, VanderVeen LA, Ali CF, Chang TK, Konakova M, Pena RL, Kanhere RS, Kirksey YM, Ji C, Wang Y, Huang J, Sweeney TD, Kantak SS, Doberstein SK. Charych DH, et al. Clin Cancer Res. 2016 Feb 1;22(3):680-90. doi: 10.1158/1078-0432.CCR-15-1631. Clin Cancer Res. 2016. PMID: 26832745 - A First-in-Human Study and Biomarker Analysis of NKTR-214, a Novel IL2Rβγ-Biased Cytokine, in Patients with Advanced or Metastatic Solid Tumors.
Bentebibel SE, Hurwitz ME, Bernatchez C, Haymaker C, Hudgens CW, Kluger HM, Tetzlaff MT, Tagliaferri MA, Zalevsky J, Hoch U, Fanton C, Aung S, Hwu P, Curti BD, Tannir NM, Sznol M, Diab A. Bentebibel SE, et al. Cancer Discov. 2019 Jun;9(6):711-721. doi: 10.1158/2159-8290.CD-18-1495. Epub 2019 Apr 15. Cancer Discov. 2019. PMID: 30988166 - NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy.
Miyazaki T, Maiti M, Hennessy M, Chang T, Kuo P, Addepalli M, Obalapur P, Sheibani S, Wilczek J, Pena R, Quach P, Cetz J, Moffett A, Tang Y, Kirk P, Huang J, Sheng D, Zhang P, Rubas W, Madakamutil L, Kivimäe S, Zalevsky J. Miyazaki T, et al. J Immunother Cancer. 2021 May;9(5):e002024. doi: 10.1136/jitc-2020-002024. J Immunother Cancer. 2021. PMID: 34001523 Free PMC article. - Interleukin-2 receptor signaling in regulatory T cell development and homeostasis.
Burchill MA, Yang J, Vang KB, Farrar MA. Burchill MA, et al. Immunol Lett. 2007 Nov 30;114(1):1-8. doi: 10.1016/j.imlet.2007.08.005. Epub 2007 Sep 14. Immunol Lett. 2007. PMID: 17936914 Free PMC article. Review. - Increasing the biological activity of IL-2 and IL-15 through complexing with anti-IL-2 mAbs and IL-15Rα-Fc chimera.
Votavova P, Tomala J, Kovar M. Votavova P, et al. Immunol Lett. 2014 May-Jun;159(1-2):1-10. doi: 10.1016/j.imlet.2014.01.017. Epub 2014 Feb 7. Immunol Lett. 2014. PMID: 24512738 Review.
Cited by
- Immune Subtypes and Characteristics of Endometrial Cancer Based on Immunogenes.
Zhang C, Xu J, Wang M, He Y, Wu Y. Zhang C, et al. Cancer Manag Res. 2024 Oct 29;16:1525-1543. doi: 10.2147/CMAR.S494838. eCollection 2024. Cancer Manag Res. 2024. PMID: 39493321 Free PMC article. - A Precision Engineered Interleukin-2 for Bolstering CD8+ T- and NK-cell Activity without Eosinophilia and Vascular Leak Syndrome in Nonhuman Primates.
Ma L, Acuff NV, Joseph IB, Ptacin JL, Caffaro CE, San Jose KM, Aerni HR, Carrio R, Byers AM, Herman RW, Pavlova Y, Pena MJ, Chen DB, Buetz C, Ismaili TK, Pham HV, Cucchetti M, Sassoon I, Koriazova LK, Leveque JA, Shawver LK, Mooney JM, Milla ME. Ma L, et al. Cancer Res Commun. 2024 Oct 1;4(10):2799-2814. doi: 10.1158/2767-9764.CRC-24-0278. Cancer Res Commun. 2024. PMID: 39320047 Free PMC article. - Harnessing IL-2 for immunotherapy against cancer and chronic infection: a historical perspective and emerging trends.
Im SJ, Lee K, Ha SJ. Im SJ, et al. Exp Mol Med. 2024 Sep;56(9):1900-1908. doi: 10.1038/s12276-024-01301-3. Epub 2024 Sep 2. Exp Mol Med. 2024. PMID: 39218982 Free PMC article. Review. - Discovery and development of ANV419, an IL-2/anti-IL-2 antibody fusion protein with potent CD8+ T and natural killer cell-stimulating capacity for cancer immunotherapy.
Murer P, Brannetti B, Rondeau JM, Petersen L, Egli N, Popp S, Regnier C, Richter K, Katopodis A, Huber C. Murer P, et al. MAbs. 2024 Jan-Dec;16(1):2381891. doi: 10.1080/19420862.2024.2381891. Epub 2024 Jul 23. MAbs. 2024. PMID: 39041287 Free PMC article. - Phase 1 first-in-human dose-escalation study of ANV419 in patients with relapsed/refractory advanced solid tumors.
Joerger M, Calvo E, Laubli H, Lopez J, Alonso G, Corral de la Fuente E, Hess D, König D, Sanchez Perez V, Bucher C, Jethwa S, Garralda E. Joerger M, et al. J Immunother Cancer. 2023 Nov 21;11(11):e007784. doi: 10.1136/jitc-2023-007784. J Immunother Cancer. 2023. PMID: 38243906 Free PMC article. Clinical Trial.
References
- McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol. 2016;34(8):833–42. doi: 10.1200/JCO.2015.63.7421 . - DOI - PubMed
- Sznol M, Longo DL. Release the Hounds! Activating the T-Cell Response to Cancer. The New England journal of medicine. 2015;372(4):374–5. doi: 10.1056/NEJMe1413488 . - DOI - PubMed
- McDermott DF, Drake CG, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al. Survival, Durable Response, and Long-Term Safety in Patients With Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. J Clin Oncol. 2015;33(18):2013–20. doi: 10.1200/JCO.2014.58.1041 ; PubMed Central PMCID: PMCPMC4517051. - DOI - PMC - PubMed
- Assal A, Kaner J, Pendurti G, Zang X. Emerging targets in cancer immunotherapy: beyond CTLA-4 and PD-1. Immunotherapy. 2015;7(11):1169–86. doi: 10.2217/imt.15.78 . - DOI - PMC - PubMed
- Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the Generation Z of Negative Checkpoint Regulators. Frontiers in immunology. 2015;6:418 doi: 10.3389/fimmu.2015.00418 ; PubMed Central PMCID: PMCPMC4544156. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
The study was funded by Nektar Therapeutics, San Francisco, California, United States of America. All authors are salaried employees of Nektar Therapeutics. DC, SK and JZ were involved in the study design, data collection and analysis. DC and JZ were involved in the decision to publish. DC, SK and UH were involved with the writing and preparation of the manuscript. Nektar Therapeutics provided support in the form of salaries for all authors, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials