Lycium barbarum Reduces Abdominal Fat and Improves Lipid Profile and Antioxidant Status in Patients with Metabolic Syndrome - PubMed (original) (raw)
Lycium barbarum Reduces Abdominal Fat and Improves Lipid Profile and Antioxidant Status in Patients with Metabolic Syndrome
Mayara Zagonel de Souza Zanchet et al. Oxid Med Cell Longev. 2017.
Abstract
Natural antioxidants present in fruits have attracted considerable interest due to their presumed safety and potential nutritional value. Even though antioxidant activities of many fruits have been reported, the effects of phytochemicals of goji berry (GB) in patients with metabolic syndrome have not been investigated. In this study, we examined anthropometric and biochemical parameters in patients with metabolic syndrome after the consumption of GB. The patients were divided into two groups, control (C) and supplemented (S), and followed up for 45 days. Participants were individually instructed to carry out a healthy diet, but additionally, an inclusion of 14 g of the natural form of goji berry in the diet during 45 days for the S group was proposed. After 45 days of study, a significant reduction in transaminases as well as an improvement in lipid profile in the S group was observed. Likewise, a significant reduction in the waist circumference of the S group was observed when compared with that of the C group, and increased glutathione and catalase levels associated with a reduction of lipid peroxidation. These results suggest that this is an effective dietary supplement for the prevention of cardiovascular diseases in individuals with metabolic syndrome.
Figures
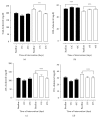
Figure 1
Lipid profile of patients with MS in the control group (C) and supplemented group (S) at the beginning, at day 15, and at day 45 after intervention. Total cholesterol (TC, (a)), HDL cholesterol (b), LDL cholesterol (c), and VLDL cholesterol (d) in patients with metabolic syndrome. Biochemical parameters were evaluated before (baseline) and 15 (15C and 15S) and 45 days (45C and 45S) after supplementation. Closed bars (control group—not supplemented) and open bars were supplemented with 14 g of goji berry daily. Values of ∗∗p < 0.01 and ∗∗∗p < 0.001 were considered statistically significant when comparing the baseline time with the end time of intervention in each group, using ANOVA followed by the Bonferroni post hoc test.
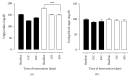
Figure 2
Serum triglycerides and fasting glycemia in patients with MS in the control group (C) and supplemented group (S) at the beginning, at day 15, and at day 45 after intervention. Evaluation of triglycerides (TG, (a)) and glucose (b) in patients with metabolic syndrome. Biochemical parameters were evaluated before (baseline) and 15 (15C and 15S) and 45 days (45C and 45S) after supplementation. Closed bars (control group—not supplemented) and open bars were supplemented with 14 g of goji berry daily. Values of ∗∗∗p < 0.001 were considered statistically significant when comparing the baseline time with the end time of intervention in each group, using ANOVA followed by the Bonferroni post hoc test.
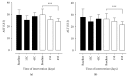
Figure 3
Hepatic enzyme patients with MS in the control group (C) and supplemented group (S) at the beginning, at day 15, and at day 45 after intervention. Evaluation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in patients with metabolic syndrome. AST (a) and ALT (b) were evaluated before (group baseline) and 15 (15C and 15S) and 45 days (45S) after supplementation with 14 g of goji berry daily. The control group (closed bars) supplemented with goji (open bars). Values of ∗∗∗p < 0.001 were considered statistically significant when comparing the baseline time with the end time of intervention in each group, using ANOVA followed by the Bonferroni post hoc test.
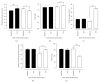
Figure 4
Oxidative stress variable patients with MS in the control group (C) and supplemented group (S) at the beginning and day 45 after intervention. Evaluation of thiobarbituric acid reactive substances (TBARS), blood reduced glutathione (GSH), total antioxidant control, catalase activity (CAT), and measurement of superoxide dismutase activity (SOD) in patients with metabolic syndrome. TBARS (a), GSH (b), total antioxidant control (c), and CzAT (d) were evaluated before (baseline) and 45 days after supplementation with 14 g of goji berry daily. Values of ∗∗p < 0.01 and ∗∗∗p < 0.001 were considered statistically significant when comparing the baseline time with the end time of intervention in each group, using ANOVA followed by the Bonferroni post hoc test.
Similar articles
- Phytochemical analysis and antioxidant activity of Lycium barbarum (Goji) cultivated in Greece.
Benchennouf A, Grigorakis S, Loupassaki S, Kokkalou E. Benchennouf A, et al. Pharm Biol. 2017 Dec;55(1):596-602. doi: 10.1080/13880209.2016.1265987. Pharm Biol. 2017. PMID: 27937034 Free PMC article. - Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults.
Amagase H, Sun B, Borek C. Amagase H, et al. Nutr Res. 2009 Jan;29(1):19-25. doi: 10.1016/j.nutres.2008.11.005. Nutr Res. 2009. PMID: 19185773 Clinical Trial. - Effect of the Lycium barbarum polysaccharides on age-related oxidative stress in aged mice.
Li XM, Ma YL, Liu XJ. Li XM, et al. J Ethnopharmacol. 2007 May 22;111(3):504-11. doi: 10.1016/j.jep.2006.12.024. Epub 2006 Dec 28. J Ethnopharmacol. 2007. PMID: 17224253 - Yeast Synthetic Biology for the Production of Lycium barbarum Polysaccharides.
Peng J, Wang L, Wang M, Du R, Qin S, Jin CY, Wei Y. Peng J, et al. Molecules. 2021 Mar 15;26(6):1641. doi: 10.3390/molecules26061641. Molecules. 2021. PMID: 33804230 Free PMC article. Review. - Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity.
Lee HS, Choi CI. Lee HS, et al. Nutrients. 2023 Sep 27;15(19):4181. doi: 10.3390/nu15194181. Nutrients. 2023. PMID: 37836464 Free PMC article. Review.
Cited by
- Wolfberry (Lycium barbarum) Consumption with a Healthy Dietary Pattern Lowers Oxidative Stress in Middle-Aged and Older Adults: A Randomized Controlled Trial.
Toh DWK, Lee WY, Zhou H, Sutanto CN, Lee DPS, Tan D, Kim JE. Toh DWK, et al. Antioxidants (Basel). 2021 Apr 7;10(4):567. doi: 10.3390/antiox10040567. Antioxidants (Basel). 2021. PMID: 33917032 Free PMC article. - The Roles of Carotenoid Consumption and Bioavailability in Cardiovascular Health.
Yao Y, Goh HM, Kim JE. Yao Y, et al. Antioxidants (Basel). 2021 Dec 11;10(12):1978. doi: 10.3390/antiox10121978. Antioxidants (Basel). 2021. PMID: 34943081 Free PMC article. Review. - A Whole Food Plant-Based Diet Is Effective for Weight Loss: The Evidence.
Greger M. Greger M. Am J Lifestyle Med. 2020 Apr 3;14(5):500-510. doi: 10.1177/1559827620912400. eCollection 2020 Sep-Oct. Am J Lifestyle Med. 2020. PMID: 32922235 Free PMC article. Review. - L. barbarum (Lycium barbarum L.) supplementation for lipid profiles in adults: A systematic review and meta-analysis of RCTs.
Zeng X, Zhao W, Wang S, Xiong H, Wu J, Ren J. Zeng X, et al. Medicine (Baltimore). 2023 Sep 29;102(39):e34952. doi: 10.1097/MD.0000000000034952. Medicine (Baltimore). 2023. PMID: 37773857 Free PMC article. - The Effects of Lycium Barbarum Polysaccharide (LBP) in a Mouse Model of Cerulein-Induced Acute Pancreatitis.
Xiong GF, Li DW, Zheng MB, Liu SC. Xiong GF, et al. Med Sci Monit. 2019 May 25;25:3880-3886. doi: 10.12659/MSM.913820. Med Sci Monit. 2019. PMID: 31127077 Free PMC article.
References
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) Jama. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical