Obesity-induces Organ and Tissue Specific Tight Junction Restructuring and Barrier Deregulation by Claudin Switching - PubMed (original) (raw)
Obesity-induces Organ and Tissue Specific Tight Junction Restructuring and Barrier Deregulation by Claudin Switching
Rizwan Ahmad et al. Sci Rep. 2017.
Abstract
Obesity increases susceptibility to multiple organ disorders, however, underlying mechanisms remain unclear. The subclinical inflammation assisted by obesity-induced gut permeability may underlie obesity-associated co-morbidities. Despite eminent clinical significance of the obesity led gut barrier abnormalities, its precise molecular regulation remains unclear. It is also unknown whether barrier deregulations, similar to the gut, characterize other vital organs in obese individuals. The claudin family of proteins is integral to the tight junction (TJ), the apical cell-cell adhesion and a key regulator of the epithelial barrier. Using comprehensive physiological and biochemical analysis of intestinal and renal tissues from high-fat diet fed mice, critical for maintaining metabolic homeostasis, this study demonstrates that profound TJ-restructuring by organ and tissue-specific claudin switching characterize obese organs. Protein expression and cellular distribution were examined. In-silico analysis further highlighted potential association of select claudins, modulated by the obesity, with signaling and metabolic pathways of pathological significance. In vitro studies using Leptin or DCA-treatment suggested causal significance of obesity-induced changes in tissue microenvironment in regulating barrier deregulations in tissue-specific manner. Overall, current findings advances our understanding of the molecular undertakings of obesity associated changes that help predispose to specific diseases and also identifies novel windows of preventive and/or therapeutic interventions.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
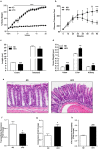
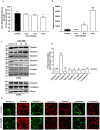
High fat diet induces obesity and associated metabolic and physiological changes in C57/BL6 mice. 8_–_10 weeks old mice were subjected to normal chow or high fat diet for 20 weeks (N = 8). (a) body weight changes in mice over the period of ND- or HFD-diet feeding; (b) Oral glucose tolerance test (OGTT); (c) Changes in colonic and intestinal length in HFD-versus ND-fed mice; (d) Changes in organ weight in HFD-versus ND-fed mice; (e) Representative images showing immune cell infiltration in mice colon with HFD- versus ND-mice. (f and g) Colonic trans-epithelial resistance and conductance across the mucosal sheet changes during HFD-mice versus control; (h) Intestinal permeability (for FITC-dextran) in HFD versus ND-mice. Values are presented as mean ± SEM ***P ≤ 0.001, **P ≤ 0.01 and *P ≤ 0.05 compared to control mice on ND.
Figure 2
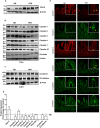
High fat diet induces restructuring of tight junction (but not adherent junction) to modulate mucosal barrier function in small intestine: Immunoblot analysis of total tissue lysate prepared using small intestine from mice fed on normal chow or high fat diet for 20 weeks (N = 4). (a) Glut-2 expression in HFD and ND-fed mice, as positive control; (b) Immunoblot analysis to determine changes in TJPs antigen-specific antibodies; (c) Immunoblot analysis to determine potential changes in AJPs (E-Cadherin and β-Catenin) in obese versus lean mice; (d) Quantitative analysis of immunoblot band intensity for respective protein; (e) Representative confocal immunofluorescent images of TJ and AJPs of small intestine from HFD- and ND-fed mice. Values are presented as mean ± SEM *P ≤ 0.05 compared to control (normal chow fed mice; ND) mice.
Figure 3
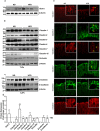
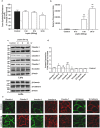
High fat diet induces colon specific restructuring of tight junction and adherent junction: Immunoblot analysis of total tissue lysate prepared using the Colon from ND- and HFD-fed mice (N = 4). (a) Glut-2 expression in HFD and ND-fed mice, as positive control; (b) Immunoblot analysis to determine changes in TJPs using antigen-specific antibodies; (c) Immunoblot analysis to determine changes in AJPs including E-cadherin and β-catenin; (d) Densitometry analysis of the immunoblot band intensity for respective protein; (e) Representative immunofluorescent images of TJ and AJPs of colon from HFD- and ND-fed mice. Values are presented as mean ± SEM **P ≤ 0.01; *P ≤ 0.05 compared to control (normal chow fed mice; ND) mice.
Figure 4
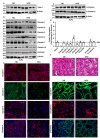
High fat diet induces contrasting (versus gut) changes in tight junction composition in renal epithelium: Immunoblot analysis of total tissue lysate prepared using the kidney from ND- and HFD-fed mice (N = 4). (a) Glut-2 expression in HFD and ND-fed mice, as positive control; (b) Immunoblot analysis to determine changes in TJPs using antigen-specific antibodies; (c) Immunoblot analysis to determine changes in AJPs including E-cadherin and β-catenin; (d) Quantitative analysis of immunoblot band intensity for respective protein; (e) Representative immunofluorescent images of specific TJPs using kidney sections from HFD- and ND-fed mice. Values are presented as mean ± SEM ***P ≤ 0.001; **P ≤ 0.01 and *P ≤ 0.05 compared to control (normal chow fed mice; ND) mice.
Figure 5
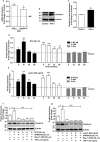
Exposure of intestinal epithelial cell (IEC) to Bile acid (DCA) modulates tight junction composition and barrier function similar to the HFD-induced tight junction restructuring in gut epithelium. Polarized monolayer of Caco-2 cells was subjected to DCA (20 µM)-treatment (in complete culture medium) for different time-points. (a) Effect of DCA-treatment upon TER across the cell monolayer; (b) Apico-basal paracellular permeability in control or DCA-treated cells as described in “materials and methods”. (c) Immunoblots analysis of total cell lysate from control and DCA treated cells by using antigen-specific antibodies; (d) Quantitative analysis of the antigen specific band intensity from immunoblot analysis; (e) Representative confocal immunoflurescent imaging to determine cellular localization of cell-adhesion proteins (TJPs and AJPs) in control and DCA-treated cells. Values are presented as mean ± SEM. ***P ≤ 0.001, **P ≤ 0.01 and *P ≤ 0.05 compared to control.
Figure 6
Leptin induced changes in intestinal epithelial barrier function are dependent on differential restructuring of tight junction than DCA-treatment. Polarized monolayer of Caco-2 cells was subjected to Leptin (500 ng)-treatment (in complete culture medium) for different time-points. (a) Effect of leptin-treatment upon trans-epithelial resistance across the cell monolayer; (b) Apico-basal paracellular permeability in control or leptin-treated cells as described in “materials and methods”. (c) Immunoblots analysis of total cell lysate from control and leptin treated cells by using antigen-specific antibodies; (d) Quantitative analysis of the antigen specific band intensity from immunoblot analysis; (e) Representative confocal immunoflurescent imaging to determine cellular localization of cell-adhesion proteins (TJPs and AJPs) in control and leptin-treated cells. Values are presented as mean ± SEM. **P ≤ 0.01 and *P ≤ 0.05 compared to control.
Figure 7
TNFα/NF-kB/JUN MAP-Kinase Signaling regulates obesity-induced increase in claudin-2 expression in intestinal epithelium. (a) qRT-PCR using total RNA isolated from control or HFD-fed mice colon; (b) Effect of TNF-α treatment upon claudin-2 expression in Caco-2 cells; (c) Quantitative analysis of the band intensity from immunoblot analysis; (d and e) Effect of leptin or DCA-treatments upon cellular signaling mechanisms and quantitative analysis of the antigen specific band intensity from immunoblot analysis; (f and g) Effect of inhibiting NF-kB or JUN MAP Kinase signaling using pathways specific inhibitors upon leptin or TNF-α induced increases in claudin-2 expressionand quantitative analysis of the antigen specific band intensity from immunoblot analysis; Values are presented as mean ± SEM. ***P ≤ 0.001, **P ≤ 0.01 and *P ≤ 0.05 compared to control.
Figure 8
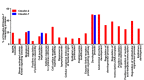
In silico analysis of claudin-2 and claudin-7 interaction in biological processes. Functional enrichment of proteins known to interact with claudin-2 and claudin-7: Many of the proteins that interact with claudin-2 encompass many biological processes including MAPK cascade and cell surface receptor signaling pathways, the proteins interacting with claudin-7 were enriched in fewer processes and were primarily involved in epithelial development.
Similar articles
- Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation.
Garcia-Hernandez V, Quiros M, Nusrat A. Garcia-Hernandez V, et al. Ann N Y Acad Sci. 2017 Jun;1397(1):66-79. doi: 10.1111/nyas.13360. Epub 2017 May 10. Ann N Y Acad Sci. 2017. PMID: 28493289 Free PMC article. Review. - Cingulin is dispensable for epithelial barrier function and tight junction structure, and plays a role in the control of claudin-2 expression and response to duodenal mucosa injury.
Guillemot L, Schneider Y, Brun P, Castagliuolo I, Pizzuti D, Martines D, Jond L, Bongiovanni M, Citi S. Guillemot L, et al. J Cell Sci. 2012 Nov 1;125(Pt 21):5005-14. doi: 10.1242/jcs.101261. Epub 2012 Sep 3. J Cell Sci. 2012. PMID: 22946046 - Changes in the expression levels of tight junction components during reconstruction of tight junction from mucosal lesion by intestinal ischemia/reperfusion.
Takizawa Y, Kishimoto H, Tomita M, Hayashi M. Takizawa Y, et al. Eur J Drug Metab Pharmacokinet. 2014 Sep;39(3):211-20. doi: 10.1007/s13318-013-0151-z. Epub 2013 Sep 8. Eur J Drug Metab Pharmacokinet. 2014. PMID: 24014129 - Changes in protein and mRNA expression levels of claudin family after mucosal lesion by intestinal ischemia/reperfusion.
Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. Takizawa Y, et al. Int J Pharm. 2012 Apr 15;426(1-2):82-89. doi: 10.1016/j.ijpharm.2012.01.023. Epub 2012 Jan 20. Int J Pharm. 2012. PMID: 22285474 - Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases.
Barmeyer C, Fromm M, Schulzke JD. Barmeyer C, et al. Pflugers Arch. 2017 Jan;469(1):15-26. doi: 10.1007/s00424-016-1914-6. Epub 2016 Nov 30. Pflugers Arch. 2017. PMID: 27904960 Review.
Cited by
- How important are fatty acids in human health and can they be used in treating diseases?
Dicks LMT. Dicks LMT. Gut Microbes. 2024 Jan-Dec;16(1):2420765. doi: 10.1080/19490976.2024.2420765. Epub 2024 Oct 27. Gut Microbes. 2024. PMID: 39462280 Free PMC article. Review. - Probiotics, prebiotics, synbiotics and other microbiome-based innovative therapeutics to mitigate obesity and enhance longevity via the gut-brain axis.
Boyajian JL, Islam P, Abosalha A, Schaly S, Thareja R, Kassab A, Arora K, Santos M, Shum-Tim C, Prakash S. Boyajian JL, et al. Microbiome Res Rep. 2024 May 17;3(3):29. doi: 10.20517/mrr.2024.05. eCollection 2024. Microbiome Res Rep. 2024. PMID: 39421246 Free PMC article. Review. - Environmental Enrichment Prevents Gut Dysbiosis Progression and Enhances Glucose Metabolism in High-Fat Diet-Induced Obese Mice.
Manzo R, Gallardo-Becerra L, Díaz de León-Guerrero S, Villaseñor T, Cornejo-Granados F, Salazar-León J, Ochoa-Leyva A, Pedraza-Alva G, Pérez-Martínez L. Manzo R, et al. Int J Mol Sci. 2024 Jun 24;25(13):6904. doi: 10.3390/ijms25136904. Int J Mol Sci. 2024. PMID: 39000013 Free PMC article. - Obesity-associated microbiomes instigate visceral adipose tissue inflammation by recruitment of distinct neutrophils.
Shantaram D, Hoyd R, Blaszczak AM, Antwi L, Jalilvand A, Wright VP, Liu J, Smith AJ, Bradley D, Lafuse W, Liu Y, Williams NF, Snyder O, Wheeler C, Needleman B, Brethauer S, Noria S, Renton D, Perry KA, Nagareddy P, Wozniak D, Mahajan S, Rana PSJB, Pietrzak M, Schlesinger LS, Spakowicz DJ, Hsueh WA. Shantaram D, et al. Nat Commun. 2024 Jun 27;15(1):5434. doi: 10.1038/s41467-024-48935-5. Nat Commun. 2024. PMID: 38937454 Free PMC article. - Immunotherapy targeting the obese white adipose tissue microenvironment: Focus on non-communicable diseases.
Priscilla L, Yoo C, Jang S, Park S, Lim G, Kim T, Lee DY. Priscilla L, et al. Bioact Mater. 2024 Feb 19;35:461-476. doi: 10.1016/j.bioactmat.2024.01.027. eCollection 2024 May. Bioact Mater. 2024. PMID: 38404641 Free PMC article.
References
- Ogden, C. L., Carroll, M. D. Fryar, C. D. & Flegal, K. M. Prevalence of Obesity Among Adults and Youth:United States, 2011–2014. NCHS Data Brief. pp. 1–8 (2015). - PubMed
- Ogden, C. L., Lamb, M. M., Carroll, M. D. & Flegal, K. M. Obesity and Socioeconomic Status in Adults: United States, 2005–2008. NCHS Data Brief; pp. 1–8 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical