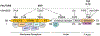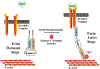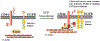Ezrin Orchestrates Signal Transduction in Airway Cells - PubMed (original) (raw)
Review
Ezrin Orchestrates Signal Transduction in Airway Cells
Lei-Miao Yin et al. Rev Physiol Biochem Pharmacol. 2018.
Abstract
Ezrin is a critical structural protein that organizes receptor complexes and orchestrates their signal transduction. In this study, we review the ezrin-meditated regulation of critical receptor complexes, including the epidermal growth factor receptor (EGFR), CD44, vascular cell adhesion molecule (VCAM), and the deleted in colorectal cancer (DCC) receptor. We also analyze the ezrin-meditated regulation of critical pathways associated with asthma, such as the RhoA, Rho-associated protein kinase (ROCK), and protein kinase A (cAMP/PKA) pathways. Mounting evidence suggests that ezrin plays a role in controlling airway cell function and potentially contributes to respiratory diseases. Ezrin can participate in asthma pathogenesis by affecting bronchial epithelium repair, T lymphocyte regulation, and the contraction of the airway smooth muscle cells. These studies provide new insights for the design of novel therapeutic strategies for asthma treatment.
Keywords: Actin-binding proteins; Airway cells; Asthma; Ezrin.
Conflict of interest statement
Competing Interests
The authors declare that they have no conflicts of interest.
Figures
Fig. 1
Structural characteristics of ezrin. Ezrin contains an N-terminal FERM domain (~300 residues), a central linker region (~200 residues), and a C-terminal ERM-associated (C-ERMAD) domain. The N-terminal domain binds to membrane receptor complexes. The linker is an α-helix with a proline-rich domain. The C-terminal C-ERMAD domain binds to F-actin. Ezrin is regulated by phosphorylation at serine-66, tyrosine-145, threonine-235, tyrosine-353, threonine-477, and threonine-567. These phosphorylations are regulated by multiple extracellular factors (EGF, TNF, and IL1β) and intracellular kinases (PKA, CDK5, Src, and Akt). FERM four-point one, ezrin, radixin, moesin, EGF epidermal growth factor, TNF tumor necrosis factor, IL1β interleukin-1β, PKA protein kinase A, CDK5 cyclin-dependent kinase 5, SRC proto-oncogene tyrosine-protein kinase Src, PIP2 phosphatidylinositol 4,5-bisphosphate, CHP1 calcineurin homologous protein-1
Fig. 2
Structural regulation of ezrin. Ezrin is normally in a dormant inactive stage with its N-terminal domain interacting and blocking the C-terminal domain. Ezrin is activated by phosphorylation. Ezrin threonine-567 is one of the most characteristic phosphorylation sites, and phosphorylation at threonine-567 causes the dissociation of the intramolecular interaction between the N- and C-terminal domains. This dissociation allows the N-terminal domain to interact with multiple receptor complexes and the C-terminal domain to interact with F-actin. P phosphorylation
Fig. 3
Proposed model of the participation of ezrin in the repair of the bronchial epithelium in asthma. EGF stimulation phosphorylates ezrin, which links CD44 to the cortical actin cytoskeleton. The interaction between CD44 and the EGFR enhances the repair efficiency of the airway epithelium. P phosphorylation, EGF epidermal growth factor, EGFR epidermal growth factor receptor
Fig. 4
Involvement of ezrin in the activation of T lymphocytes in asthma. At the immunological synapse, a TCR signal activates Rac1, which dephosphorylates ezrin, while binding to PIP2 re-phosphorylates ezrin. This transient dephosphorylation and re-phosphorylation of ezrin can change the cellular morphology of T lymphocytes. Ezrin interacts with CD43 during T lymphocyte activation at the DPC. P phosphorylation, TCR T cell receptor, PIP2 phosphatidylinositol 4,5-bisphosphate, APC antigen-presenting cells, Rac1 ras-related C3 botulinum toxin substrate 1, DPC distal pole complex
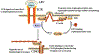
Fig. 5
Possible mechanism of the ezrin-mediated regulation of the contraction and relaxation of airway smooth muscle cells. The PKA-Ezrin complex is the effector of β2-adrenergic receptor signaling in the regulation of the contraction and relaxation of ASMCs. Phosphorylated ezrin binds to Rho-GDI, which enhances the activation of RhoA and induces the relaxation of ASMCs. P phosphorylation, AC adenylate cyclase, cAMP cyclic adenosine monophosphate, PKA protein kinase A, GDP guanosine diphosphate, GDI GDP dissociation inhibitor, GEF guanine nucleotide exchange factor, GTP guanosine-5′-triphosphate
Similar articles
- Brain natriuretic peptide modulates calcium homeostasis and epidermal growth factor receptor gene signalling in asthmatic airways smooth muscle cells.
Orlandi A, Calzetta L, Doldo E, Tarquini C, Matera MG, Passeri D. Orlandi A, et al. Pulm Pharmacol Ther. 2015 Apr;31:51-4. doi: 10.1016/j.pupt.2015.02.005. Epub 2015 Feb 23. Pulm Pharmacol Ther. 2015. PMID: 25722070 - [The expression and clinical significance of RhoA, Ezrin and CD44 in laryngeal squamous cell carcinoma].
Xie YX, Shang XL, Fan J. Xie YX, et al. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017 Feb 5;31(3):191-194. doi: 10.13201/j.issn.1001-1781.2017.03.006. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017. PMID: 29871220 Chinese. - TGFβ and IGF1R signaling activates protein kinase A through differential regulation of ezrin phosphorylation in colon cancer cells.
Leiphrakpam PD, Brattain MG, Black JD, Wang J. Leiphrakpam PD, et al. J Biol Chem. 2018 May 25;293(21):8242-8254. doi: 10.1074/jbc.RA117.001299. Epub 2018 Mar 29. J Biol Chem. 2018. PMID: 29599290 Free PMC article. - Mechanisms underlying the pathogenesis of hyper-contractility of bronchial smooth muscle in allergic asthma.
Sakai H, Suto W, Kai Y, Chiba Y. Sakai H, et al. J Smooth Muscle Res. 2017;53(0):37-47. doi: 10.1540/jsmr.53.37. J Smooth Muscle Res. 2017. PMID: 28484126 Free PMC article. Review. - [Advances of the Role of Ezrin in Migration and Invasion of Breast Cancer Cells].
Long ZY, Wang TH. Long ZY, et al. Sheng Li Ke Xue Jin Zhan. 2016 Feb;47(1):21-6. Sheng Li Ke Xue Jin Zhan. 2016. PMID: 27424401 Review. Chinese.
Cited by
- Ezrin is essential for the entry of Japanese encephalitis virus into the human brain microvascular endothelial cells.
Liu YG, Chen Y, Wang X, Zhao P, Zhu Y, Qi Z. Liu YG, et al. Emerg Microbes Infect. 2020 Dec;9(1):1330-1341. doi: 10.1080/22221751.2020.1757388. Emerg Microbes Infect. 2020. PMID: 32538298 Free PMC article. - Editorial: Evolution, Emerging Functions and Structure of Actin-Binding Proteins.
Yin LM, Schnoor M, Jun CD. Yin LM, et al. Front Cell Dev Biol. 2021 Dec 10;9:819300. doi: 10.3389/fcell.2021.819300. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957129 Free PMC article. No abstract available. - Cartilaginous Extracellular Matrix Enriched with Human Gingival Mesenchymal Stem Cells Derived "Matrix Bound Extracellular Vesicles" Enabled Functional Reconstruction of Tracheal Defect.
Zeng T, Yuan P, Liang L, Zhang X, Zhang H, Wu W. Zeng T, et al. Adv Sci (Weinh). 2022 Jan;9(2):e2102735. doi: 10.1002/advs.202102735. Epub 2021 Nov 28. Adv Sci (Weinh). 2022. PMID: 34841733 Free PMC article. - Promotion of the occurrence of endometrioid carcinoma by S100 calcium binding protein P.
Zhang D, Chen X, Xia H, Wang L, Zhao H, Xu B, Zhang A, Zhang W. Zhang D, et al. BMC Cancer. 2020 Sep 3;20(1):845. doi: 10.1186/s12885-020-07350-x. BMC Cancer. 2020. PMID: 32883230 Free PMC article.
References
- Abbattiscianni AC, Favia M, Mancini MT, Cardone RA, Guerra L, Monterisi S, Castellani S, Laselva O, Di Sole F, Conese M, Zaccolo M, Casavola V (2016) Correctors of mutant CFTR enhance subcortical cAMP-PKA signaling through modulating ezrin phosphorylation and cytoskeleton organization. J Cell Sci 129:1128–1140 - PubMed
- Allenspach EJ, Cullinan P, Tong J, Tang Q, Tesciuba AG, Cannon JL, Takahashi SM, Morgan R, Burkhardt JK, Sperling AI (2001) ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15:739–750 - PubMed
- Ammar B, Alice G (2016) ET-1 enhances EGFR phosphorylation via Src activation in asthmatic airway smooth muscle. In: A33. Airway smooth muscle. American Thoracic Society international conference abstracts, American Thoracic Society, p A1347
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB (2000) Inhibition of RhoA by p120 catenin. Nat Cell Biol 2:637–644 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous