Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target - PubMed (original) (raw)
Review
Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target
Amadou K S Camara et al. Front Physiol. 2017.
Abstract
Mitochondria are the key source of ATP that fuels cellular functions, and they are also central in cellular signaling, cell division and apoptosis. Dysfunction of mitochondria has been implicated in a wide range of diseases, including neurodegenerative and cardiac diseases, and various types of cancer. One of the key proteins that regulate mitochondrial function is the voltage-dependent anion channel 1 (VDAC1), the most abundant protein on the outer membrane of mitochondria. VDAC1 is the gatekeeper for the passages of metabolites, nucleotides, and ions; it plays a crucial role in regulating apoptosis due to its interaction with apoptotic and anti-apoptotic proteins, namely members of the Bcl-2 family of proteins and hexokinase. Therefore, regulation of VDAC1 is crucial not only for metabolic functions of mitochondria, but also for cell survival. In fact, multiple lines of evidence have confirmed the involvement of VDAC1 in several diseases. Consequently, modulation or dysregulation of VDAC1 function can potentially attenuate or exacerbate pathophysiological conditions. Understanding the role of VDAC1 in health and disease could lead to selective protection of cells in different tissues and diverse diseases. The purpose of this review is to discuss the role of VDAC1 in the pathogenesis of diseases and as a potentially effective target for therapeutic management of various pathologies.
Keywords: Alzheimer's disease; cardiac ischemia/reperfusion; hexokinase; mitochondria; molecular dynamics; neoplastic diseases; post-translational modification; voltage dependent anion channel.
Figures
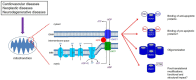
Figure 1
VDAC1 is expressed on the outer mitochondrial membrane (OMM). Together with ANT1 on the inner mitochondrial membrane (IMM) and mitochondrial creatine kinase (mCK), the VDAC1-ANT1-mCK complex regulates the exchange of ATP and ADP between the mitochondria and cytosol. VDAC1 functions as a receptor for anti- and pro-apoptotic proteins and, consequently, contributes to cell survival and cell death. *Bax/Bak binding to VDAC1 has not been definitively shown as various studies report on conflicting results; on the other hand, Bax/Bak binding to VDAC2 has been supported by more consistent results. VDAC1 oligomerization can result in increased permeability of the OMM; however, the mechanism that leads to apoptosis has not been clearly defined. Various types of post-translational modificantions (PTMs) of VDAC1 have been reported, although their impact on channel function and subsequently on mitochondrial function is not well understood.
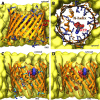
Figure 2
Structure of human VDAC1 in membrane. (A) Sideview and (B) cytoplasmic topview of the beta-barrel, with basic residues in blue sticks, acidic E73 in red sticks, and ATP in spheres. (C) Significant ATP-interacting residues along permeation pathway (data adopted from Choudhary et al., 2014). (D) Currently confirmed phosphorylation sites in human VDAC1 (data adopted from Martel et al., 2014), positions of phospho-serines and phospho-threonine highlighted in red spheres. C-terminal part of the beta-barrel is omitted in (C,D) for clarity.
Similar articles
- The mitochondrial voltage-dependent anion channel 1 in tumor cells.
Shoshan-Barmatz V, Ben-Hail D, Admoni L, Krelin Y, Tripathi SS. Shoshan-Barmatz V, et al. Biochim Biophys Acta. 2015 Oct;1848(10 Pt B):2547-75. doi: 10.1016/j.bbamem.2014.10.040. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25448878 Review. - Voltage-Dependent Anion Channel 1 As an Emerging Drug Target for Novel Anti-Cancer Therapeutics.
Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A, Arif T. Shoshan-Barmatz V, et al. Front Oncol. 2017 Jul 31;7:154. doi: 10.3389/fonc.2017.00154. eCollection 2017. Front Oncol. 2017. PMID: 28824871 Free PMC article. Review. - Key regions of VDAC1 functioning in apoptosis induction and regulation by hexokinase.
Shoshan-Barmatz V, Zakar M, Rosenthal K, Abu-Hamad S. Shoshan-Barmatz V, et al. Biochim Biophys Acta. 2009 May;1787(5):421-30. doi: 10.1016/j.bbabio.2008.11.009. Epub 2008 Nov 27. Biochim Biophys Acta. 2009. PMID: 19094960 - Decoding Cancer through Silencing the Mitochondrial Gatekeeper VDAC1.
Arif T, Shteinfer-Kuzmine A, Shoshan-Barmatz V. Arif T, et al. Biomolecules. 2024 Oct 15;14(10):1304. doi: 10.3390/biom14101304. Biomolecules. 2024. PMID: 39456237 Free PMC article. Review. - VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease.
Shoshan-Barmatz V, Krelin Y, Shteinfer-Kuzmine A. Shoshan-Barmatz V, et al. Cell Calcium. 2018 Jan;69:81-100. doi: 10.1016/j.ceca.2017.06.007. Epub 2017 Jun 23. Cell Calcium. 2018. PMID: 28712506 Review.
Cited by
- Mitochondrial Complex I and β-Amyloid Peptide Interplay in Alzheimer's Disease: A Critical Review of New and Old Little Regarded Findings.
Atlante A, Valenti D. Atlante A, et al. Int J Mol Sci. 2023 Nov 3;24(21):15951. doi: 10.3390/ijms242115951. Int J Mol Sci. 2023. PMID: 37958934 Free PMC article. Review. - Recent progress in the use of mitochondrial membrane permeability transition pore in mitochondrial dysfunction-related disease therapies.
Cui Y, Pan M, Ma J, Song X, Cao W, Zhang P. Cui Y, et al. Mol Cell Biochem. 2021 Jan;476(1):493-506. doi: 10.1007/s11010-020-03926-0. Epub 2020 Sep 30. Mol Cell Biochem. 2021. PMID: 33000352 Review. - Regulation of Cell Death by Mitochondrial Transport Systems of Calcium and Bcl-2 Proteins.
Naumova N, Šachl R. Naumova N, et al. Membranes (Basel). 2020 Oct 21;10(10):299. doi: 10.3390/membranes10100299. Membranes (Basel). 2020. PMID: 33096926 Free PMC article. Review. - Assessing the role of residue E73 and lipid headgroup charge in VDAC1 voltage gating.
Queralt-Martín M, Bergdoll L, Jacobs D, Bezrukov SM, Abramson J, Rostovtseva TK. Queralt-Martín M, et al. Biochim Biophys Acta Bioenerg. 2019 Jan;1860(1):22-29. doi: 10.1016/j.bbabio.2018.11.001. Epub 2018 Nov 6. Biochim Biophys Acta Bioenerg. 2019. PMID: 30412693 Free PMC article. - Interactions between NLRP3 inflammasome and glycolysis in macrophages: New insights into chronic inflammation pathogenesis.
Yu Q, Guo M, Zeng W, Zeng M, Zhang X, Zhang Y, Zhang W, Jiang X, Yu B. Yu Q, et al. Immun Inflamm Dis. 2022 Mar;10(3):e581. doi: 10.1002/iid3.581. Epub 2021 Dec 13. Immun Inflamm Dis. 2022. PMID: 34904398 Free PMC article. Review.
References
- Alavian K. N., Beutner G., Lazrove E., Sacchetti S., Park H. A., Licznerski P., et al. . (2014). An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. U.S.A. 111, 10580–10585. 10.1073/pnas.1401591111 - DOI - PMC - PubMed
- Aldakkak M., Camara A. K., Heisner J. S., Yang M., Stowe D. F. (2011). Ranolazine reduces Ca2+ overload and oxidative stress and improves mitochondrial integrity to protect against ischemia reperfusion injury in isolated hearts. Pharmacol. Res. 64, 381–392. 10.1016/j.phrs.2011.06.018 - DOI - PMC - PubMed
Publication types
Grants and funding
- R01 HL131673/HL/NHLBI NIH HHS/United States
- U54 GM087519/GM/NIGMS NIH HHS/United States
- P01 GM066730/GM/NIGMS NIH HHS/United States
- R01 GM086749/GM/NIGMS NIH HHS/United States
- P41 GM104601/GM/NIGMS NIH HHS/United States
- R01 GM101048/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources