A Recombinant 47-kDa Outer Membrane Protein Induces an Immune Response against Orientia tsutsugamushi Strain Boryong - PubMed (original) (raw)
A Recombinant 47-kDa Outer Membrane Protein Induces an Immune Response against Orientia tsutsugamushi Strain Boryong
Sangho Choi et al. Am J Trop Med Hyg. 2017 Jul.
Abstract
We investigated the 47-kDa outer membrane protein (OMP), which is a periplasmic serine protease and an antigenic major surface protein of Orientia tsutsugamushi, as a vaccine candidate. We developed a conventional subunit vaccine expressing recombinant 47-kDa OMP (rec47) and a DNA vaccine (p47). In mouse immunization experiments, intranasal immunization with rec47 alone or with rec47 plus heat-labile enterotoxin B subunit from Escherichia coli or plus cholera toxin (CT) as adjuvants induced a higher amount of rec47-specific antibodies than intramuscular immunization with p47 alone or with p47 plus pBOOST2-samIRF7/3 (pB) as adjuvant. Moreover, the combination of rec47 and CT induced a strong cellular immune response to 47-kDa OMP, as demonstrated by a spleen cell proliferation assay, and also induced Th1- and Th2-type cytokine production, as demonstrated by a cytokine enzyme-linked immunosorbent assay. Intranasal immunization with rec47 plus CT was the most effective method for the induction of humoral and cell-mediated immune responses. Furthermore, relatively strong protection against homologous O. tsutsugamushi strain Boryong challenge was observed in mice immunized with rec47 plus CT. Therefore, 47-kDa OMP is an attractive candidate for developing a prophylactic vaccine against scrub typhus by O. tsutsugamushi infection.
Figures
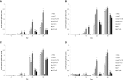
Figure 1.
Humoral immune response upon the different treatments, as measured in sera from immunized mice. 47-kDa outer membrane protein–specific immunoglobulins including total (A) IgG, (B) IgG1, (C) IgG2a, and (D) IgA were measured by indirect enzyme-linked immunosorbent assay after every immunization. Statistically significant differences between the PBS control group and each recombinant protein immunization group are indicated with * (P < 0.05) or ** (P < 0.01). Statistically significant differences between the pVAX1 control group and each DNA vaccination group are indicated with † (P < 0.05) or †† (P < 0.01).
Figure 2.
Spleen cell proliferation assay to measure cell-mediated immunization. The isolated spleen cells were restimulated with 1 μg of rec47. Cell viability was measured as the percentage of viable cells at 24, 48, and 72 hours after restimulation. Statistically significant differences between the PBS control group and each recombinant protein immunization group are indicated with * (P < 0.05) or ** (P < 0.01). Statistically significant differences between the pVAX1 control group and each DNA vaccination group are indicated with † (P < 0.05) or †† (P < 0.01).
Figure 3.
Cytokine release in immunized mice sera. After the third immunization, the concentration of distinct Th1- and Th2-type cytokines in mouse serum was measured by using a multiplex assay. (A) interferon gamma (IFN-γ), (B) interleukin (IL)-2, (C) IL-4, (D) IL-5, (E) IL-6, (F) IL-10, and (G) IL-12 (p70). Statistically significant differences between the PBS control group and each recombinant protein immunization group are indicated with * (P < 0.05) or ** (P < 0.01). Statistically significant differences between the pVAX1 control group and each DNA vaccination group are indicated with † (P < 0.05) or †† (P < 0.01).
Figure 4.
Cytokine release from spleen cells isolated from immunized mice. The isolated spleen cells were restimulated with 1 μg of rec47. Cytokines were measured at 24 hours after restimulation using an enzyme-linked immunosorbent assay. (A) interferon gamma (IFN-γ), (B) tumor necrosis factor (TNF)-α, (C) interleukin (IL)-2, (D) IL-5, (E) IL-6, and (F) IL-10. Statistically significant differences between the PBS control group and each recombinant protein immunization group are indicated with * (P < 0.05) or ** (P < 0.01). Statistically significant differences between the pVAX1 control group and each DNA vaccination group are indicated with † (P < 0.05) or †† (P < 0.01).
Figure 5.
Evaluation of the protection against homologous challenge in immunized mice. Orientia tsutsugamushi strain Boryong was injected intraperitoneally into mice immunized with rec47 alone or rec47 plus LTB/CT and into mice immunized with p47 alone or p47 and pB, and their respective control treatments. The mortality was monitored daily for 2 weeks. The survival rate was calculated as the ratio of the number of living mice to the total number of challenged mice in a group. Statistically significant differences between the PBS control group and each recombinant protein immunization group are indicated with * (P < 0.05). Statistically significant differences between the pVAX1 control group and each DNA vaccination group are indicated with † (P < 0.05).
Similar articles
- Protective immunity of 56-kDa type-specific antigen of Orientia tsutsugamushi causing scrub typhus.
Choi S, Jeong HJ, Ju YR, Gill B, Hwang KJ, Lee J. Choi S, et al. J Microbiol Biotechnol. 2014 Dec 28;24(12):1728-35. doi: 10.4014/jmb.1407.07048. J Microbiol Biotechnol. 2014. PMID: 25112312 - Recombinant 56-kilodalton major outer membrane protein antigen of Orientia tsutsugamushi Shanxi and its antigenicity.
Chen WJ, Niu DS, Zhang XY, Chen ML, Cui H, Wei WJ, Wen BH, Chen XR. Chen WJ, et al. Infect Immun. 2003 Aug;71(8):4772-9. doi: 10.1128/IAI.71.8.4772-4779.2003. Infect Immun. 2003. PMID: 12874360 Free PMC article. - Immunization with a recombinant antigen composed of conserved blocks from TSA56 provides broad genotype protection against scrub typhus.
Kim HI, Ha NY, Kim G, Min CK, Kim Y, Yen NTH, Choi MS, Cho NH. Kim HI, et al. Emerg Microbes Infect. 2019;8(1):946-958. doi: 10.1080/22221751.2019.1632676. Emerg Microbes Infect. 2019. PMID: 31237478 Free PMC article. - Approaches to vaccines against Orientia tsutsugamushi.
Valbuena G, Walker DH. Valbuena G, et al. Front Cell Infect Microbiol. 2013 Jan 4;2:170. doi: 10.3389/fcimb.2012.00170. eCollection 2012. Front Cell Infect Microbiol. 2013. PMID: 23316486 Free PMC article. Review. - Orientia tsutsugamushi infection: overview and immune responses.
Seong SY, Choi MS, Kim IS. Seong SY, et al. Microbes Infect. 2001 Jan;3(1):11-21. doi: 10.1016/s1286-4579(00)01352-6. Microbes Infect. 2001. PMID: 11226850 Review.
Cited by
- Intranasal Vaccination with Outer-Membrane Protein of Orientia tsutsugamushi induces Protective Immunity Against Scrub Typhus.
Park SM, Gu MJ, Ju YJ, Cheon IS, Hwang KJ, Gill B, Shim BS, Jeong HJ, Son YM, Choi S, Jeung W, Han SH, Chu H, Yun CH. Park SM, et al. Immune Netw. 2020 Dec 3;21(2):e14. doi: 10.4110/in.2021.21.e14. eCollection 2021 Apr. Immune Netw. 2020. PMID: 33996170 Free PMC article. - In silico construction of a multi-epitope vaccine (RGME-VAC/ATS-1) against the Rickettsia genus using immunoinformatics.
Felice AG, Rodrigues TCV, Marques PH, Zen FL, Lemes MR, Trevisan RO, Andrade BS, de Oliveira CJF, Azevedo VAC, Tiwari S, Soares SC. Felice AG, et al. Mem Inst Oswaldo Cruz. 2025 Mar 21;120:e240201. doi: 10.1590/0074-02760240201. eCollection 2025. Mem Inst Oswaldo Cruz. 2025. PMID: 40136144 Free PMC article. - Dysregulated Th1 Immune and Vascular Responses in Scrub Typhus Pathogenesis.
Soong L. Soong L. J Immunol. 2018 Feb 15;200(4):1233-1240. doi: 10.4049/jimmunol.1701219. J Immunol. 2018. PMID: 29431689 Free PMC article. Review. - Vaccine development: obligate intracellular bacteria new tools, old pathogens: the current state of vaccines against obligate intracellular bacteria.
van Schaik EJ, Fratzke AP, Gregory AE, Dumaine JE, Samuel JE. van Schaik EJ, et al. Front Cell Infect Microbiol. 2024 Mar 19;14:1282183. doi: 10.3389/fcimb.2024.1282183. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38567021 Free PMC article. Review. - Dual-Antigen Subunit Vaccine Nanoparticles for Scrub Typhus.
Park J, Zhang Z, Belinskaya T, Tsoras AN, Chao CC, Jiang L, Champion JA. Park J, et al. Pathogens. 2023 Nov 25;12(12):1390. doi: 10.3390/pathogens12121390. Pathogens. 2023. PMID: 38133275 Free PMC article.
References
- Chattopadhyay S, Richards AL, 2007. Scrub typhus vaccines: past history and recent developments. Hum Vaccin 3: 73–80. - PubMed
- Seong SY, Choi MS, Kim IS, 2001. Orientia tsutsugamushi infection: overview and immune responses. Microbes Infect 3: 11–21. - PubMed
- Watt G, Parola P, 2003. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis 16: 429–436. - PubMed
- Yu Y, Wen B, Wen B, Niu D, Chen M, Qiu L, 2005. Induction of protective immunity against scrub typhus with a 56-kilodalton recombinant antigen fused with a 47-kilodalton antigen of Orientia tsutsugamushi Karp. Am J Trop Med Hyg 72: 458–464. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases