Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke - PubMed (original) (raw)
doi: 10.1016/j.omtm.2017.06.005. eCollection 2017 Sep 15.
Chikako Nito 1, Kota Sowa 1 2, Satoshi Suda 1, Yasuhiro Nishiyama 1, Aki Nakamura-Takahashi 2 3, Yuko Nitahara-Kasahara 2 4, Kiwamu Imagawa 5, Tohru Hirato 5, Masayuki Ueda 1, Kazumi Kimura 1, Takashi Okada 2 4
Affiliations
- PMID: 28725658
- PMCID: PMC5502709
- DOI: 10.1016/j.omtm.2017.06.005
Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke
Masataka Nakajima et al. Mol Ther Methods Clin Dev. 2017.
Abstract
Interleukin (IL)-10 is a contributing factor to neuroprotection of mesenchymal stem cell (MSC) transplantation after ischemic stroke. Our aim was to increase therapeutic effects by combining MSCs and ex vivo IL-10 gene transfer with an adeno-associated virus (AAV) vector using a rat transient middle cerebral artery occlusion (MCAO) model. Sprague-Dawley rats underwent 90 min MCAO followed by intravenous administration of MSCs alone or IL-10 gene-transferred MSCs (MSC/IL-10) at 0 or 3 hr after ischemia reperfusion. Infarct lesions, neurological deficits, and immunological analyses were performed within 7 days after MCAO. 0-hr transplantation of MSCs alone and MSC/IL-10 significantly reduced infarct volumes and improved motor function. Conversely, 3-hr transplantation of MSC/IL-10, but not MSCs alone, significantly reduced infarct volumes (p < 0.01) and improved motor function (p < 0.01) compared with vehicle groups at 72 hr and 7 days after MCAO. Immunological analysis showed that MSC/IL-10 transplantation significantly inhibits microglial activation and pro-inflammatory cytokine expression compared with MSCs alone. Moreover, overexpressing IL-10 suppressed neuronal degeneration and improved survival of engrafted MSCs in the ischemic hemisphere. These results suggest that overexpressing IL-10 enhances the neuroprotective effects of MSC transplantation by anti-inflammatory modulation and thereby supports neuronal survival during the acute ischemic phase.
Keywords: adeno-associated virus; focal cerebral ischemia; gene transfer; interleukin-10; intravenous transplantation; mesenchymal stem cell; neuroprotection.
Figures
Figure 1
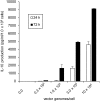
ELISA Quantification of IL-10 In Vitro IL-10 concentrations were determined in 24 or 72 hr culture supernatants of IL-10 gene-transferred MSCs. 1 × 105 MSCs were transfected with dsAAV1-CAG-rat IL-10 vg at 0, 0.3, 1, 3, and 10 × 105 per cell. Data are presented as mean ± SD. n = 3 for each group.
Figure 2
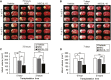
Reduction of Infarct Volumes as Detected by TTC Staining (A and B) Representative photographs showing TTC staining at 72 hr (A) and 7 days (B) after MCAO in vehicle, MSC, or MSC/IL-10-transplanted reperfusion groups at 0 or 3 hr. (C and D) Quantitative analysis of infarct volumes in each group at 72 hr (C) and 7 days (D) after MCAO. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group.
Figure 3
Improvement of Neurological Score and Motor Function (A and B) Abnormal posture (A) and hemiparesis (B) assessment at 7 days after MCAO in vehicle, MSC, or MSC/IL-10-transplanted reperfusion groups at 0 or 3 hr. Box plots indicate the median and interquartile range, and whiskers indicate the maximum and minimum values (*p < 0.05, **p < 0.01). n = 8 for each group. (C and D) Rotarod performance (C) and forelimb grip strength (D) assessment at 7 days after MCAO in vehicle, MSC, or MSC/IL-10-transplanted reperfusion groups at 0 or 3 hours. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 8 for each group.
Figure 4
Immunohistochemical Staining for Iba-1 and TNF-α in the Cortical Ischemic Boundary Zone (A) The red-lined square on the brain map shows the cortical ischemic boundary zone. The black area represents the ischemic lesion. (B) Immunohistochemical examination of Iba-1 at 24 and 72 hr after MCAO. Scale bar, 100 μm. (C) Number of immunopositive cells. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group. (D) Immunohistochemical examination of TNF-α at 24 and 72 hr after MCAO. Scale bar, 40 μm. (E) Number of immunopositive cells. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group.
Figure 5
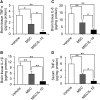
Effect of MSC Transplantation on ELISA expression of TNF-α, IL-1β, and IL-6 in Brain Tissue Extracts or Serum (A–C) Quantification of TNF-α (A), IL-1β (B), and IL-6 (C) expression in ischemic hemisphere extracts at 72 hr after MCAO. (D) Quantification of TNF-α expression in serum at 72 hr after MCAO. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 4 for each group.
Figure 6
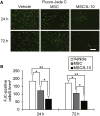
Fluoro-Jade C Staining of Neuronal Degeneration in the Cortical Ischemic Boundary Zone (A) Immunohistochemical examination of Fluoro-Jade C at 24 and 72 hr after MCAO. Scale bar, 100 μm. (B) Number of immunopositive cells. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group.
Figure 7
IL-10 Expression in the Ischemic Hemisphere and Serum (A) Immunohistochemical examination of IL-10 at 24 and 72 hr after MCAO. Scale bar, 100 μm. (B) Number of immunopositive cells. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 5 for each group. (C) ELISA quantification of IL-10 expression in the brain at 24 and 72 hr after MCAO. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 4 for each group. (D) ELISA quantification of IL-10 expression in serum at 24 and 72 hr after MCAO. Data are presented as mean ± SD (*p < 0.05, **p < 0.01). n = 4 for each group.
Figure 8
Tracking, Biodistribution, and Quantification of Engrafted MSCs (A) Tracking of engrafted MSCs and MSC/IL-10 in the ischemic hemisphere by PKH26 labeling at 72 hr and 14 days after MCAO. Fluorescent microscopy detected red fluorescent engrafted cells. Scale bar, 100 μm. (B) Immunofluorescence staining of rat IL-10 (green) and human mitochondria (red) in the ischemic hemisphere of MSC/IL-10-transplanted rats at 72 hr after MCAO. Arrows in the merged image show rat IL-10 expression of engrafted MSC/IL-10. Scale bar, 100 μm. (C and D) Biodistribution and quantification of engrafted MSCs and MSC/IL-10 at 72 hr (C) and 7 days (D) after MCAO using Alu-based real-time PCR. Data are presented as mean ± SD (*p < 0.05). n = 4 for each group.
Similar articles
- Progress in Mesenchymal Stem Cell Therapy for Ischemic Stroke.
Guo Y, Peng Y, Zeng H, Chen G. Guo Y, et al. Stem Cells Int. 2021 Jun 15;2021:9923566. doi: 10.1155/2021/9923566. eCollection 2021. Stem Cells Int. 2021. PMID: 34221026 Free PMC article. Review. - Intra-Arterial Transplantation of Allogeneic Mesenchymal Stem Cells Mounts Neuroprotective Effects in a Transient Ischemic Stroke Model in Rats: Analyses of Therapeutic Time Window and Its Mechanisms.
Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, Sasaki T, Sasada S, Shinko A, Wakamori T, Okazaki M, Kondo A, Agari T, Borlongan CV, Date I. Toyoshima A, et al. PLoS One. 2015 Jun 15;10(6):e0127302. doi: 10.1371/journal.pone.0127302. eCollection 2015. PLoS One. 2015. PMID: 26075717 Free PMC article. - Adrenomedullin enhances therapeutic potency of mesenchymal stem cells after experimental stroke in rats.
Hanabusa K, Nagaya N, Iwase T, Itoh T, Murakami S, Shimizu Y, Taki W, Miyatake K, Kangawa K. Hanabusa K, et al. Stroke. 2005 Apr;36(4):853-8. doi: 10.1161/01.STR.0000157661.69482.76. Epub 2005 Mar 3. Stroke. 2005. PMID: 15746464 - Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits.
Zhu Y, Guan YM, Huang HL, Wang QS. Zhu Y, et al. Acta Pharmacol Sin. 2014 May;35(5):585-91. doi: 10.1038/aps.2014.9. Epub 2014 Apr 14. Acta Pharmacol Sin. 2014. PMID: 24727940 Free PMC article. - Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke.
Hsuan YC, Lin CH, Chang CP, Lin MT. Hsuan YC, et al. Brain Behav. 2016 Aug 3;6(10):e00526. doi: 10.1002/brb3.526. eCollection 2016 Oct. Brain Behav. 2016. PMID: 27781140 Free PMC article. Review.
Cited by
- Immune Cells After Ischemic Stroke Onset: Roles, Migration, and Target Intervention.
Ao LY, Yan YY, Zhou L, Li CY, Li WT, Fang WR, Li YM. Ao LY, et al. J Mol Neurosci. 2018 Nov;66(3):342-355. doi: 10.1007/s12031-018-1173-4. Epub 2018 Oct 1. J Mol Neurosci. 2018. PMID: 30276612 Review. - Progress in Mesenchymal Stem Cell Therapy for Ischemic Stroke.
Guo Y, Peng Y, Zeng H, Chen G. Guo Y, et al. Stem Cells Int. 2021 Jun 15;2021:9923566. doi: 10.1155/2021/9923566. eCollection 2021. Stem Cells Int. 2021. PMID: 34221026 Free PMC article. Review. - Mesenchymal Stem Cell Derived Extracellular Vesicles for Repairing the Neurovascular Unit after Ischemic Stroke.
Davis C, Savitz SI, Satani N. Davis C, et al. Cells. 2021 Mar 31;10(4):767. doi: 10.3390/cells10040767. Cells. 2021. PMID: 33807314 Free PMC article. Review. - Synthetic Extracellular Matrices as a Toolbox to Tune Stem Cell Secretome.
Liu K, Veenendaal T, Wiendels M, Ruiz-Zapata AM, van Laar J, Kyranas R, Enting H, van Cranenbroek B, Koenen HJPM, Mihaila SM, Oosterwijk E, Kouwer PHJ. Liu K, et al. ACS Appl Mater Interfaces. 2020 Dec 23;12(51):56723-56730. doi: 10.1021/acsami.0c16208. Epub 2020 Dec 11. ACS Appl Mater Interfaces. 2020. PMID: 33305561 Free PMC article. - The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Therapeutic Efficacy.
García-Bernal D, García-Arranz M, Yáñez RM, Hervás-Salcedo R, Cortés A, Fernández-García M, Hernando-Rodríguez M, Quintana-Bustamante Ó, Bueren JA, García-Olmo D, Moraleda JM, Segovia JC, Zapata AG. García-Bernal D, et al. Front Cell Dev Biol. 2021 Mar 16;9:650664. doi: 10.3389/fcell.2021.650664. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33796536 Free PMC article. Review.
References
- Asadi H., Dowling R., Yan B., Wong S., Mitchell P. Advances in endovascular treatment of acute ischaemic stroke. Intern. Med. J. 2015;45:798–805. - PubMed
- Yoo J., Kim H.S., Hwang D.Y. Stem cells as promising therapeutic options for neurological disorders. J. Cell. Biochem. 2013;114:743–753. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources