Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer - PubMed (original) (raw)
Review
Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer
Anna Watermann et al. Nanomaterials (Basel). 2017.
Abstract
Even though cancer treatment has improved over the recent decades, still more specific and effective treatment concepts are mandatory. Surgical removal is not always possible, metastases are challenging and chemo- and radiotherapy can not only have severe side-effects but also resistances may occur. To cope with these challenges more efficient therapies with fewer side-effects are required. One promising approach is the use of drug delivery vehicles. Here, mesoporous silica nanoparticles (MSN) are discussed as biodegradable drug carrier to improve efficacy and reduce side-effects. MSN excellently fulfill the criteria for nanoparticulate carriers: their distinct structure allows high loading capacity and a plethora of surface modifications. MSN synthesis permits fine-tuning of particle and pore sizes. Moreover, drug release can be tailored through various gatekeeper systems which are for example pH-sensitive or redox-sensitive. Furthermore, MSN can either enter tumors passively by the enhanced permeability and retention effect or can be actively targeted by various ligands. PEGylation prolongs circulation time and availability. A huge advantage of MSN is their explicitly low toxic profile in vivo. Yet, clinical translation remains challenging. Overall, mesoporous silica nanoparticles are a promising tool for innovative, more efficient and safer cancer therapies.
Keywords: biocompatibility; drug delivery; mesoporous silica nanoparticles; tumor targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
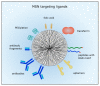
Figure 1
Ligands for active tumor targeting. MSN can be coated with poly (ethylene glycol) (PEG) to prolong circulation time. Small molecules such as folic acid are often used for active targeting. Different peptides with the arginine-glycine-aspartic acid (RGD) motif or proteins such as transferrin were also employed for tumor targeting. Moreover, aptamers, antibodies or antibody fragments are utilized to target membrane-receptors which are commonly overexpressed in cancer cells.
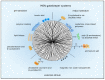
Figure 2
MSN gatekeeper systems to control drug release. Drug release can be regulated by internal stimuli such as pH decrease or reduction by glutathione or by external stimuli. PH-sensitive systems respond to acidic pH in the tumor microenvironment and in the endolysosomal system. Several examples are presented here such as pseudorotaxan encircled by β-cyclodextrin, tannic acid, polymer and lipid coatings. Several capping structures are linked to the MSN via disulfide bonds which are reduced by glutathione intracellularly. Then the pore blocking agents such as β-cyclodextrin, cystamine, poly-(β-aminoesters) and ammonium salt are released and the drugs can escape the nanoparticle. External stimuli such as light and magnetism are utilized to control drug release, too. Photolabile coumarin encircled by β-cyclodextrin is cleaved from the MSN by light or a magnetic field stimulates iron oxide nanoparticles to release the encapsulated drugs.
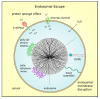
Figure 3
Endosomal escape mechanisms. After MSN were taken up by endocytosis, an endosomal escape is mandatory for drug efficacy. Coating with cationic polymers such as polyethyleneimine or poly-(β-aminoesters) induces the proton sponge effect. The proton concentration increases during hydrolysis which leads to an increase in membrane potential and influx of counter-ions such as chloride ions. Finally, osmotic swelling by water inflow bursts the endosome and the MSN with its cargo is delivered into the cytosol. Also, fusogenic peptides such as KALA or zwitterionic co-lipids such as dioleoyl-phosphatidylethanolamine (DOPE) can destabilize the endosomal membrane resulting in MSN release.
Similar articles
- Facile Synthesis of Three Types of Mesoporous Silica Microspheres as Drug Delivery Carriers and their Sustained-Release Properties.
Zhu Y, Wang B, Chen J, He J, Qiu X. Zhu Y, et al. Curr Drug Deliv. 2023;20(9):1337-1350. doi: 10.2174/1567201819666220616121602. Curr Drug Deliv. 2023. PMID: 35713141 - Carboxyl-functionalized mesoporous silica nanoparticles for the controlled delivery of poorly water-soluble non-steroidal anti-inflammatory drugs.
Gou K, Wang Y, Guo X, Wang Y, Bian Y, Zhao H, Guo Y, Pang Y, Xie L, Li S, Li H. Gou K, et al. Acta Biomater. 2021 Oct 15;134:576-592. doi: 10.1016/j.actbio.2021.07.023. Epub 2021 Jul 17. Acta Biomater. 2021. PMID: 34280558 - pH-triggered sustained release of arsenic trioxide by polyacrylic acid capped mesoporous silica nanoparticles for solid tumor treatment in vitro and in vivo.
Xiao X, Liu Y, Guo M, Fei W, Zheng H, Zhang R, Zhang Y, Wei Y, Zheng G, Li F. Xiao X, et al. J Biomater Appl. 2016 Jul;31(1):23-35. doi: 10.1177/0885328216637211. Epub 2016 Apr 7. J Biomater Appl. 2016. PMID: 27059495 - Multifunctional Mesoporous Silica Nanoparticles for Cancer Therapy and Imaging.
Dilnawaz F. Dilnawaz F. Curr Med Chem. 2019;26(31):5745-5763. doi: 10.2174/0929867325666180501101044. Curr Med Chem. 2019. PMID: 29714137 Review. - Multimodal Decorations of Mesoporous Silica Nanoparticles for Improved Cancer Therapy.
Barui S, Cauda V. Barui S, et al. Pharmaceutics. 2020 Jun 8;12(6):527. doi: 10.3390/pharmaceutics12060527. Pharmaceutics. 2020. PMID: 32521802 Free PMC article. Review.
Cited by
- Theranostic Mesoporous Silica Nanoparticles Loaded With a Curcumin-Naphthoquinone Conjugate for Potential Cancer Intervention.
Freidus LG, Kumar P, Marimuthu T, Pradeep P, Choonara YE. Freidus LG, et al. Front Mol Biosci. 2021 May 20;8:670792. doi: 10.3389/fmolb.2021.670792. eCollection 2021. Front Mol Biosci. 2021. PMID: 34095225 Free PMC article. - Targeting the pancreatic tumor microenvironment by plant-derived products and their nanoformulations.
Saadh MJ, Mustafa MA, Malathi H, Ahluwalia G, Kaur S, Al-Dulaimi MAAH, Alubiady MHS, Zain Al-Abdeen SH, Shakier HG, Ali MS, Ahmad I, Abosaoda MK. Saadh MJ, et al. Med Oncol. 2024 Jul 13;41(8):201. doi: 10.1007/s12032-024-02443-0. Med Oncol. 2024. PMID: 39001987 Review. - Enhanced Fluorescence of N-Acetyl-β-D-Glucosaminidase Activity by ZnO Quantum Dots for Early Stage Mastitis Evaluation.
Nirala NR, Shtenberg G. Nirala NR, et al. Front Chem. 2019 Nov 8;7:754. doi: 10.3389/fchem.2019.00754. eCollection 2019. Front Chem. 2019. PMID: 31788469 Free PMC article. - Identifying the Molecular Mechanisms and Types of Cell Death Induced by _bio_- and _pyr_-Silica Nanoparticles in Endothelial Cells.
Solarska-Ściuk K, Adach K, Fijałkowski M, Haczkiewicz-Leśniak K, Kulus M, Olbromski M, Glatzel-Plucińska N, Szelest O, Bonarska-Kujawa D. Solarska-Ściuk K, et al. Int J Mol Sci. 2022 May 4;23(9):5103. doi: 10.3390/ijms23095103. Int J Mol Sci. 2022. PMID: 35563494 Free PMC article. - Concept Design, Development and Preliminary Physical and Chemical Characterization of Tamoxifen-Guided-Mesoporous Silica Nanoparticles.
Day CM, Sweetman MJ, Hickey SM, Song Y, Liu Y, Zhang N, Plush SE, Garg S. Day CM, et al. Molecules. 2021 Jan 4;26(1):219. doi: 10.3390/molecules26010219. Molecules. 2021. PMID: 33406699 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous