Mechanical strain sensing implicated in cell shape recovery in Escherichia coli - PubMed (original) (raw)
Mechanical strain sensing implicated in cell shape recovery in Escherichia coli
Felix Wong et al. Nat Microbiol. 2017.
Abstract
The shapes of most bacteria are imparted by the structures of their peptidoglycan cell walls, which are determined by many dynamic processes that can be described on various length scales ranging from short-range glycan insertions to cellular-scale elasticity1-11. Understanding the mechanisms that maintain stable, rod-like morphologies in certain bacteria has proved to be challenging due to an incomplete understanding of the feedback between growth and the elastic and geometric properties of the cell wall3,4,12-14. Here, we probe the effects of mechanical strain on cell shape by modelling the mechanical strains caused by bending and differential growth of the cell wall. We show that the spatial coupling of growth to regions of high mechanical strain can explain the plastic response of cells to bending4 and quantitatively predict the rate at which bent cells straighten. By growing filamentous Escherichia coli cells in doughnut-shaped microchambers, we find that the cells recovered their straight, native rod-shaped morphologies when released from captivity at a rate consistent with the theoretical prediction. We then measure the localization of MreB, an actin homologue crucial to cell wall synthesis, inside confinement and during the straightening process, and find that it cannot explain the plastic response to bending or the observed straightening rate. Our results implicate mechanical strain sensing, implemented by components of the elongasome yet to be fully characterized, as an important component of robust shape regulation in E. coli.
Conflict of interest statement
Competing Financial Interests
The authors declare no competing financial interests.
Figures
Figure 1. Three theories for cellular shape regulation.
a, The processivity of glycan insertions provides a robust, built-in mechanism for curvature decay, but even in the infinitely processive limit a cell remains self-similar. b, A geometry-dependent growth mechanism predicts an oppositely-bent shape once an applied hydrodynamic drag force is extinguished, which was not observed in previous experiments. c, A mechanical strain-dependent growth rate can explain both the elastic snapback shown in b and straightening, and the straightening rate can be quantitatively predicted. (Left) Simulated equilibrium configurations of a bent cylinder (top) and a toroidal shell (bottom) subject to an internal pressure, which respectively describe the cell states under a bending force (Phase 1) and in the absence of a bending force (Phase 2). The mesh, processed using finite-element software, is colored by the variational areal strain δA. Like the differential growth, δA flips signs between the two phases. (Right) The simulated, normalized variational areal strain for c = 0.1 and varying values of dimensionless pressure η are plotted against the azimuthal angle θ, along with the linear theory prediction, for a Phase 2 cell. Values of η are calculated using the radii of deformed states. The Poisson ratio is taken to be ν = 0.3 and the remaining simulation parameters are detailed in the Supplementary Methods.
Figure 2. Areal strain-dependent PG elongation quantitatively predicts shape recovery dynamics.
a, MreB molecules are modeled as points that move circumferentially along the PG mesh with a spot velocity v and unbind as a Poisson process with rate 1_/τ_. The growth at an angle θ at a given time depends on the number of initiated glycan strands also at θ, which in turn depends on the strain profile of the cell in the past (see also the growth equation in Supplementary Note 2). b, A sensitivity analysis of the theoretically predicted straightening rate for several material properties, assuming a large MreB processivity of M τ = 6 radians. The elastic snapback and material parameters determine the value of the areal strain-coupling parameter α self-consistently, as discussed in Supplementary Note 2. The predicted straightening rate is consistent with the experimental data shown in Fig. 3c. c, Numerical solutions of the growth equation agree with the theoretical prediction for the straightening rate. Here the aspect ratio is defined as the product of arclength and curvature, L(t)C(t), which does not decay without areal strain-coupling in the limit of infinite processivity (dashed lines; see also Supplementary Video 11). The normalized time is defined with units of 1_/λ_ = td/ ln(2). d, There is an intermediate value of the processivity, measured in units of time for a constant rate of PG subunit insertion, for which mechanical strain-sensing confers the largest effect on straightening. Empirical values of MreB processivity, intriguingly, lie close to the optimal value at which a cell straightens fastest.
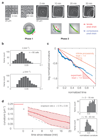
Figure 3. Quantitative analysis of cellular straightening dynamics.
a, In our experiments, filamentous E. coli cells were grown in confined, toroidal microchambers of diameter d = 8 μ_m. An elastic snapback was observed upon removal, after which the cells recovered their straight, rod-like morphologies over time. Images shown correspond to before (0 min and 90 min) and after (2 min, 10 min, 20 min, and 30 min) microchamber release. b, Histograms for the instantaneous growth rate λ = dL/(Ldt) and instantaneous straightening rate μ = −_dC/(Cdt) for 60 E. coli cells. c, A plot of the log normalized curvature, defined as ln(C(t)/C(2 min)), as a function of time since release (in units of td/ ln(2)) for all 60 cells in Phase 2. A 50-point moving average filter along the temporal direction was applied to smooth out the data. The population-averaged straightening-to-growth ratio is 〈μ_〉/〈λ〉 = 1.8, which cannot be explained by an infinite processivity of PG synthesis. The slower rate of decrease of the log normalized curvature for large times may be an artifact of substrate pinning for large cells. d, (Left) Extrapolating the population-averaged curvature to the time of microchamber release at t = 0 yields a mean elastic snapback ratio of κ = 0.78 ± 0.09. Shaded areas denote values within one standard deviation of the population mean. The gray dashed lines denote exponential fits to values which are one standard deviation away from the population average. (Right) The distributions of aggregated normalized curvatures C(t)/C_(0) at times t = 2–16 min and t = 16–30 min.
Figure 4. MreB-msfGFP fusion cells exhibit MreB enrichment at negative Gaussian curvature, but MreB enrichment alone cannot explain straightening.
a, MreB is predominantly localized at the inner edges of filamentous MreB-msfGFP E. coli cells. Shown is the MreB-msfGFP intensity ratio between the inner and outer cell edges, measured in confinement, after release from confinement, and 30 minutes into recovery. For each condition, open circles indicate averages obtained from at least 15 cells per replicate experiment, as detailed in the Methods section. Filled circles indicated averages over replicates and error bars indicate standard deviations between replicates. The solid curves indicate the predicted MreB intensity ratio for a model of constant MreB initiation rate and different processivity times τ, assuming a spot velocity of v = 5 nm/s (model a in the legend; for details, see Supplementary Note 2). The dashed line indicates the MreB intensity ratio that would be required to account for the observed straightening ratio of 1.8 within a model where cell-wall synthesis depends only on MreB localization (model b in the legend). Thus, MreB localization is consistent with a model of constant initiation and finite processivity, and the observed intensity ratio is not sufficient to account for cell straightening. b, The MreB-msfGFP intensities decrease as a function of signed centerline curvature where the inner and outer edges of a cell correspond to negative and positive values of centerline curvature, respectively. Error bars denote standard deviations, as in a.
Similar articles
- Rod-like bacterial shape is maintained by feedback between cell curvature and cytoskeletal localization.
Ursell TS, Nguyen J, Monds RD, Colavin A, Billings G, Ouzounov N, Gitai Z, Shaevitz JW, Huang KC. Ursell TS, et al. Proc Natl Acad Sci U S A. 2014 Mar 18;111(11):E1025-34. doi: 10.1073/pnas.1317174111. Epub 2014 Feb 18. Proc Natl Acad Sci U S A. 2014. PMID: 24550515 Free PMC article. - The Rcs stress response and accessory envelope proteins are required for de novo generation of cell shape in Escherichia coli.
Ranjit DK, Young KD. Ranjit DK, et al. J Bacteriol. 2013 Jun;195(11):2452-62. doi: 10.1128/JB.00160-13. Epub 2013 Mar 29. J Bacteriol. 2013. PMID: 23543719 Free PMC article. - Mechanical control of bacterial cell shape.
Jiang H, Si F, Margolin W, Sun SX. Jiang H, et al. Biophys J. 2011 Jul 20;101(2):327-35. doi: 10.1016/j.bpj.2011.06.005. Biophys J. 2011. PMID: 21767484 Free PMC article. - How to Build a Bacterial Cell: MreB as the Foreman of E. coli Construction.
Shi H, Bratton BP, Gitai Z, Huang KC. Shi H, et al. Cell. 2018 Mar 8;172(6):1294-1305. doi: 10.1016/j.cell.2018.02.050. Cell. 2018. PMID: 29522748 Free PMC article. Review. - MreB: pilot or passenger of cell wall synthesis?
White CL, Gober JW. White CL, et al. Trends Microbiol. 2012 Feb;20(2):74-9. doi: 10.1016/j.tim.2011.11.004. Epub 2011 Dec 7. Trends Microbiol. 2012. PMID: 22154164 Review.
Cited by
- A central role for PBP2 in the activation of peptidoglycan polymerization by the bacterial cell elongation machinery.
Rohs PDA, Buss J, Sim SI, Squyres GR, Srisuknimit V, Smith M, Cho H, Sjodt M, Kruse AC, Garner EC, Walker S, Kahne DE, Bernhardt TG. Rohs PDA, et al. PLoS Genet. 2018 Oct 18;14(10):e1007726. doi: 10.1371/journal.pgen.1007726. eCollection 2018 Oct. PLoS Genet. 2018. PMID: 30335755 Free PMC article. - Cell biomechanics and mechanobiology in bacteria: Challenges and opportunities.
Harper CE, Hernandez CJ. Harper CE, et al. APL Bioeng. 2020 Apr 1;4(2):021501. doi: 10.1063/1.5135585. eCollection 2020 Jun. APL Bioeng. 2020. PMID: 32266323 Free PMC article. Review. - Microfluidic techniques for mechanical measurements of biological samples.
Salipante PF. Salipante PF. Biophys Rev (Melville). 2023 Jan 20;4(1):011303. doi: 10.1063/5.0130762. eCollection 2023 Mar. Biophys Rev (Melville). 2023. PMID: 38505816 Free PMC article. Review. - Homeostasis of cytoplasmic crowding by cell wall fluidization and ribosomal counterions.
Basan M, Mukherjee A, Huang Y, Oh S, Sanchez C, Chang YF, Liu X, Bradshaw G, Benites N, Paulsson J, Kirschner M, Sung Y, Elgeti J. Basan M, et al. Res Sq [Preprint]. 2024 Apr 19:rs.3.rs-4138690. doi: 10.21203/rs.3.rs-4138690/v1. Res Sq. 2024. PMID: 38699329 Free PMC article. Preprint. - Subcellular Organization: A Critical Feature of Bacterial Cell Replication.
Surovtsev IV, Jacobs-Wagner C. Surovtsev IV, et al. Cell. 2018 Mar 8;172(6):1271-1293. doi: 10.1016/j.cell.2018.01.014. Cell. 2018. PMID: 29522747 Free PMC article. Review.
References
- Cabeen MT, Jacobs-Wagner C. Bacterial cell shape. Nat Rev Microbiol. 2005;3:601–610. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources