Sex differences in hippocampal damage, cognitive impairment, and trophic factor expression in an animal model of an alcohol use disorder - PubMed (original) (raw)
Sex differences in hippocampal damage, cognitive impairment, and trophic factor expression in an animal model of an alcohol use disorder
Mark E Maynard et al. Brain Struct Funct. 2018 Jan.
Abstract
Compared to men, women disproportionally experience alcohol-related organ damage, including brain damage, and while men remain more likely to drink and to drink heavily, there is cause for concern because women are beginning to narrow the gender gap in alcohol use disorders. The hippocampus is a brain region that is particularly vulnerable to alcohol damage, due to cell loss and decreased neurogenesis. In the present study, we examined sex differences in hippocampal damage following binge alcohol. Consistent with our prior findings, we found a significant binge-induced decrement in dentate gyrus (DG) granule neurons in the female DG. However, in the present study, we found no significant decrement in granule neurons in the male DG. We show that the decrease in granule neurons in females is associated with both spatial navigation impairments and decreased expression of trophic support molecules. Finally, we show that post-binge exercise is associated with an increase in trophic support and repopulation of the granule neuron layer in the female hippocampus. We conclude that sex differences in alcohol-induced hippocampal damage are due in part to a paucity of trophic support and plasticity-related signaling in females.
Keywords: Alcohol binge; Cognitive deficits; Neurodegeneration; Neurorestoration; Sex differences; Trophic support.
Conflict of interest statement
Conflict of interest: The authors declare that they have no conflict of interest.
Figures
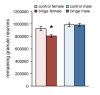
Figure 1. Sex-dependent effect of binge alcohol on the dentate gyrus
Binge alcohol significantly decreased the number of remaining granule neurons in the dentate gyrus of female rats, but not males. *p < 0.05 significantly different from control.
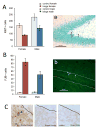
Figure 2. Binge alcohol decreased cell proliferation and increased cell death in the dentate gyrus
Cell proliferation was significantly decreased by binge alcohol in both sexes (A; arrowheads in panel a indicate Ki67+ cells). Cell death (B) was increased by binge alcohol in the dentate gyrus of both sexes (FJB+ cells indicated in panel b by arrowheads). Cell death in the dentate gyrus did not appear to be apoptotic in nature, as we found virtually no TUNEL+ cells in binged animals (C; left panel shows positive control with arrowhead pointing to TUNEL+ cell).
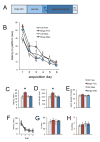
Figure 3. Sex-dependent effect of binge alcohol on spatial navigation
Panel A shows the timeline of behavioral analysis following binge alcohol. Binge alcohol had a significant, sex-dependent effect on acquisition of the spatial task. All groups ultimately learned the platform location, but binged males and females were initially slower to find it compared to same-sex controls (B). Binged males more rapidly achieved control performance, compared to binged females. The significant Sex x Diet interaction is apparent for both latency (C) and path length (D), with binged females being significantly different from all other groups. There was no difference between groups for swim speed (E). There were no differences between groups for reversal learning (F–H). *p < 0.05 significantly different from all other groups.
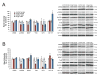
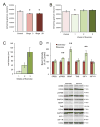
Figure 4. Sex differences in protein expression during binge exposure
Expression of proteins associated with trophic support was assessed after 24 (A) and 48 (B) hours of binge exposure, Binge alcohol caused a decrease in pCREB, BDNF and trkB at 24 hours in females, whereas males showed a decrease in pCREB only. Binged males showed an increase in IGFR at 24 hours, but binged females did not show this until 48 hours, at which point IGF-1 was downregulated in binged animals of both sexes. At 48 hours, binged males had normalized expression of pCREB, but binged females did not. *p < 0.05 significantly different from same sex control.
Figure 5. Sex differences in protein expression after binge exposure
Expression of proteins associated with trophic support was assessed 8 hours after the end of binge exposure. Binged females continue to show a decrease in pCREB and at this point CREB is decreased as well. They also show decreased BDNF as well as the trkB receptor and IGF-1. In contrast, binged males only show a decrease in BDNF. *p < 0.05 significantly different from same sex control.
Figure 6. Exercise-driven repair is associated with increased expression of plasticity-related proteins
The binge-induced decrement in remaining granule neurons in the dentate gyrus of the female hippocampus is detectable immediately following binge (Binge 0) as well as 4 weeks later (Binge 28) (A). However, if animals exercise for at least 2 weeks post-binge, the number of granule neurons is normalized (B). Panel C depicts the total distance covered by animals that ran for 1, 2 or 3 weeks, beginning after 7 days of abstinence. One week of post-binge exercise increased the expression of pCREB, BDNF and IGF-1 (D). *p < 0.05 significantly different from control; **p < 0.05 significantly different from sedentary.
Similar articles
- Neural Perturbations Associated With Recurrent Binge Alcohol in Male and Female Rats.
West RK, Rodgers SP, Leasure JL. West RK, et al. Alcohol Clin Exp Res. 2021 Feb;45(2):365-374. doi: 10.1111/acer.14529. Epub 2020 Dec 29. Alcohol Clin Exp Res. 2021. PMID: 33295022 Free PMC article. - Impact of exercise and a complex environment on hippocampal dendritic morphology, Bdnf gene expression, and DNA methylation in male rat pups neonatally exposed to alcohol.
Boschen KE, McKeown SE, Roth TL, Klintsova AY. Boschen KE, et al. Dev Neurobiol. 2017 Jun;77(6):708-725. doi: 10.1002/dneu.22448. Epub 2016 Sep 21. Dev Neurobiol. 2017. PMID: 27597545 Free PMC article. - Exercise neuroprotection in a rat model of binge alcohol consumption.
Leasure JL, Nixon K. Leasure JL, et al. Alcohol Clin Exp Res. 2010 Mar 1;34(3):404-14. doi: 10.1111/j.1530-0277.2009.01105.x. Epub 2009 Dec 17. Alcohol Clin Exp Res. 2010. PMID: 20028365 Free PMC article. - Exercise-driven restoration of the alcohol-damaged brain.
West RK, Najjar LZ, Leasure JL. West RK, et al. Int Rev Neurobiol. 2019;147:219-267. doi: 10.1016/bs.irn.2019.07.003. Epub 2019 Aug 6. Int Rev Neurobiol. 2019. PMID: 31607356 Review. - Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling.
Phillips C, Baktir MA, Srivatsan M, Salehi A. Phillips C, et al. Front Cell Neurosci. 2014 Jun 20;8:170. doi: 10.3389/fncel.2014.00170. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24999318 Free PMC article. Review.
Cited by
- Exercise leads to sex-specific recovery of behavior and pathological AD markers following adolescent ethanol exposure in the TgF344-AD model.
Reitz NL, Nunes PT, Savage LM. Reitz NL, et al. Front Behav Neurosci. 2024 Aug 1;18:1448691. doi: 10.3389/fnbeh.2024.1448691. eCollection 2024. Front Behav Neurosci. 2024. PMID: 39148897 Free PMC article. - Cognitive impairment among alcohol treatment service users in South Wales: an exploratory examination of typologies of behaviour, impairment, and service attendance.
Davies NH, Lewis J, John B, Quelch D, Roderique-Davies G. Davies NH, et al. Front Psychiatry. 2024 Jul 17;15:1377039. doi: 10.3389/fpsyt.2024.1377039. eCollection 2024. Front Psychiatry. 2024. PMID: 39091457 Free PMC article. - Treadmill Exercise Reshapes Cortical Astrocytic and Neuronal Activity to Improve Motor Learning Deficits Under Chronic Alcohol Exposure.
Liu L, Luo L, Wei JA, Xu X, So KF, Zhang L. Liu L, et al. Neurosci Bull. 2024 Sep;40(9):1287-1298. doi: 10.1007/s12264-024-01226-x. Epub 2024 May 28. Neurosci Bull. 2024. PMID: 38807019 Free PMC article. - Neuroimmune Activation and Microglia Reactivity in Female Rats Following Alcohol Dependence.
Melbourne JK, Wooden JI, Carlson ER, Anasooya Shaji C, Nixon K. Melbourne JK, et al. Int J Mol Sci. 2024 Jan 28;25(3):1603. doi: 10.3390/ijms25031603. Int J Mol Sci. 2024. PMID: 38338883 Free PMC article. - Omega-3 Recovers Cannabinoid 1 Receptor Expression in the Adult Mouse Brain after Adolescent Binge Drinking.
Martín-Llorente A, Serrano M, Bonilla-Del Río I, Lekunberri L, Ocerin G, Puente N, Ramos A, Rico-Barrio I, Gerrikagoitia I, Grandes P. Martín-Llorente A, et al. Int J Mol Sci. 2023 Dec 10;24(24):17316. doi: 10.3390/ijms242417316. Int J Mol Sci. 2023. PMID: 38139145 Free PMC article.
References
- Agartz I, Brag S, Franck J, Hammarberg A, Okugawa G, Svinhufvud K, Bergman H. MR volumetry during acute alcohol withdrawal and abstinence: a descriptive study. Alcohol and alcoholism. 2003;38:71–78. - PubMed
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Archives of general psychiatry. 1999;56:356–363. - PubMed
- Alfonso-Loeches S, Pascual M, Guerri C. Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 2013;311:27–34. - PubMed
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behavioral neuroscience. 2002;116:884–901. - PubMed
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural brain research. 2003;139:197–213. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous