Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database - PubMed (original) (raw)
Observational Study
. 2018 Jul;67(7):1306-1316.
doi: 10.1136/gutjnl-2017-314057. Epub 2017 Jul 28.
Pål Møller 1 2 3, Inge Bernstein 5 6, Elke Holinski-Feder 7 8, Paulo Sala 9, D Gareth Evans 10 11, Annika Lindblom 12, Finlay Macrae 13 14, Ignacio Blanco 15, Rolf H Sijmons 16, Jacqueline Jeffries 17, Hans F A Vasen 18, John Burn 19, Sigve Nakken 2, Eivind Hovig 2 20 21, Einar Andreas Rødland 2, Kukatharmini Tharmaratnam 22, Wouter H de Vos Tot Nederveen Cappel 23, James Hill 24, Juul T Wijnen 25, Mark A Jenkins 26, Kate Green 10 11, Fiona Lalloo 10 11, Lone Sunde 6 27 28, Miriam Mints 29, Lucio Bertario 9, Marta Pineda 15, Matilde Navarro 15, Monika Morak 7 8, Laura Renkonen-Sinisalo 4 30, Mev Dominguez Valentin 1 2, Ian M Frayling, John-Paul Plazzer 13, Kirsi Pylvanainen 31, Maurizio Genuardi [ 32](#full-view-affiliation-32 "Institute of Genomic Medicine, "A. Gemelli" Faculty of Medicine, Catholic University of the Sacred Heart, Rome, Italy."), Jukka-Pekka Mecklin 33, Gabriela Moeslein, Julian R Sampson 17, Gabriel Capella 15 3; Mallorca Group
Affiliations
- PMID: 28754778
- PMCID: PMC6031262
- DOI: 10.1136/gutjnl-2017-314057
Observational Study
Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database
Pål Møller et al. Gut. 2018 Jul.
Erratum in
- Correction: Cancer risk and survival in path _MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database.
[No authors listed] [No authors listed] Gut. 2020 Jun;69(6):e4. doi: 10.1136/gutjnl-2017-314057corr1. Gut. 2020. PMID: 32381558 Free PMC article. No abstract available.
Abstract
Background: Most patients with path_MMR gene variants (Lynch syndrome (LS)) now survive both their first and subsequent cancers, resulting in a growing number of older patients with LS for whom limited information exists with respect to cancer risk and survival.
Objective and design: This observational, international, multicentre study aimed to determine prospectively observed incidences of cancers and survival in path_MMR carriers up to 75 years of age.
Results: 3119 patients were followed for a total of 24 475 years. Cumulative incidences at 75 years (risks) for colorectal cancer were 46%, 43% and 15% in path_ MLH1, path_ MSH2 and path_ MSH6 carriers; for endometrial cancer 43%, 57% and 46%; for ovarian cancer 10%, 17% and 13%; for upper gastrointestinal (gastric, duodenal, bile duct or pancreatic) cancers 21%, 10% and 7%; for urinary tract cancers 8%, 25% and 11%; for prostate cancer 17%, 32% and 18%; and for brain tumours 1%, 5% and 1%, respectively. Ovarian cancer occurred mainly premenopausally. By contrast, upper gastrointestinal, urinary tract and prostate cancers occurred predominantly at older ages. Overall 5-year survival for prostate cancer was 100%, urinary bladder 93%, ureter 85%, duodenum 67%, stomach 61%, bile duct 29%, brain 22% and pancreas 0%. Path_PMS2 carriers had lower risk for cancer.
Conclusion: Carriers of different path_MMR variants exhibit distinct patterns of cancer risk and survival as they age. Risk estimates for counselling and planning of surveillance and treatment should be tailored to each patient's age, gender and path_MMR variant. We have updated our open-access website www.lscarisk.org to facilitate this.
Keywords: HNPCC syndrome; cancer prevention; clinical trials; colorectal cancer screening; inherited cancers.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: John Burn has a patent for high speed low cost tumour profiling pending to John Burn and QuantuMDx.
Figures
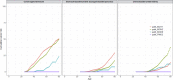
Figure 1
Cumulative incidences of colorectal cancer, upper gastrointestinal cancer (including stomach, duodenum, bile duct, gall bladder and pancreas) and urinary tract cancer (not including prostate).
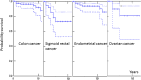
Figure 2
Kaplan-Meier estimates of 10-year crude survival following colon cancer, sigmoid-rectal cancer, endometrial cancer or ovarian cancer that was diagnosed before 65 years of age after censoring for upper gastrointestinal or brain tumours. Solid line indicates point values; dotted lines indicate 95% CIs.
Comment in
- The study of Lynch syndrome in a special population reveals a strong founder effect and an unusual mutational mechanism in familial adenomatous polyposis.
Siraj AK, Masoodi T, Bu R, Parvathareddy SK, Siraj S, Alassiri A, Al-Dayel F, Alkuraya FS, Al-Kuraya KS. Siraj AK, et al. Gut. 2020 Nov;69(11):2048-2049. doi: 10.1136/gutjnl-2019-320511. Epub 2020 Jan 10. Gut. 2020. PMID: 31924657 Free PMC article. No abstract available.
Similar articles
- Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database.
Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I, Sijmons R, Jeffries J, Vasen H, Burn J, Nakken S, Hovig E, Rødland EA, Tharmaratnam K, de Vos Tot Nederveen Cappel WH, Hill J, Wijnen J, Jenkins M, Green K, Lalloo F, Sunde L, Mints M, Bertario L, Pineda M, Navarro M, Morak M, Renkonen-Sinisalo L, Frayling IM, Plazzer JP, Pylvanainen K, Genuardi M, Mecklin JP, Möslein G, Sampson JR, Capella G; Mallorca Group (http://mallorca-group.org). Møller P, et al. Gut. 2017 Sep;66(9):1657-1664. doi: 10.1136/gutjnl-2016-311403. Epub 2016 Jun 3. Gut. 2017. PMID: 27261338 Free PMC article. - Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database.
Møller P, Seppälä T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I, Sijmons R, Jeffries J, Vasen H, Burn J, Nakken S, Hovig E, Rødland EA, Tharmaratnam K, de Vos Tot Nederveen Cappel WH, Hill J, Wijnen J, Green K, Lalloo F, Sunde L, Mints M, Bertario L, Pineda M, Navarro M, Morak M, Renkonen-Sinisalo L, Frayling IM, Plazzer JP, Pylvanainen K, Sampson JR, Capella G, Mecklin JP, Möslein G; Mallorca Group (http://mallorca-group.eu). Møller P, et al. Gut. 2017 Mar;66(3):464-472. doi: 10.1136/gutjnl-2015-309675. Epub 2015 Dec 9. Gut. 2017. PMID: 26657901 Free PMC article. - Commentary on "Risks of primary extracolonic cancers following colorectal cancer in Lynch syndrome." Win AK, Lindor NM, Young JP, Macrae FA, Young GP, Williamson E, Parry S, Goldblatt J, Lipton L, Winship I, Leggett B, Tucker KM, Giles GG, Buchanan DD, Clendenning M, Rosty C, Arnold J, Levine AJ, Haile RW, Gallinger S, Le Marchand L, Newcomb PA, Hopper JL, Jenkins MA, Centre for Molecular, Environmental, Genetic and Analytic Epidemiology, Melbourne School of Population Health, The University of Melbourne, Victoria, Australia: J Natl Cancer Inst 2012;104(18):1363-72 [Epub 2012 Aug 28].
See WA. See WA. Urol Oncol. 2013 Jul;31(5):716. doi: 10.1016/j.urolonc.2013.03.013. Urol Oncol. 2013. PMID: 23796201 - The Prospective Lynch Syndrome Database reports enable evidence-based personal precision health care.
Møller P. Møller P. Hered Cancer Clin Pract. 2020 Mar 14;18:6. doi: 10.1186/s13053-020-0138-0. eCollection 2020. Hered Cancer Clin Pract. 2020. PMID: 32190163 Free PMC article. Review. - Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications.
Bonis PA, Trikalinos TA, Chung M, Chew P, Ip S, DeVine DA, Lau J. Bonis PA, et al. Evid Rep Technol Assess (Full Rep). 2007 May;(150):1-180. Evid Rep Technol Assess (Full Rep). 2007. PMID: 17764220 Free PMC article. Review.
Cited by
- The utility of evaluating mismatch repair proteins in endometrial carcinoma: an experience from a tertiary referral centre in North India.
Jain E, Prasad S, Dhar A, Kini L, Sharma S, Dewan A. Jain E, et al. Pathologica. 2021 Apr;113(2):115-120. doi: 10.32074/1591-951X-129. Pathologica. 2021. PMID: 34042092 Free PMC article. - Is a colorectal neoplasm diagnosis a trigger to change dietary and other lifestyle habits for persons with Lynch syndrome? A prospective cohort study.
Brouwer JGM, Snellen M, Bisseling TM, Koornstra JJ, Vasen HFA, Kampman E, van Duijnhoven FJB. Brouwer JGM, et al. Fam Cancer. 2021 Apr;20(2):125-135. doi: 10.1007/s10689-020-00201-5. Epub 2020 Aug 8. Fam Cancer. 2021. PMID: 32770331 Free PMC article. - Genetic Variation in PEAR1, Cardiovascular Outcomes and Effects of Aspirin in a Healthy Elderly Population.
Lewis JP, Riaz M, Xie S, Polekhina G, Wolfe R, Nelson M, Tonkin AM, Reid CM, Murray AM, McNeil JJ, Shuldiner AR, Lacaze P. Lewis JP, et al. Clin Pharmacol Ther. 2020 Dec;108(6):1289-1298. doi: 10.1002/cpt.1959. Epub 2020 Jul 20. Clin Pharmacol Ther. 2020. PMID: 32562573 Free PMC article. Clinical Trial. - Mismatch Repair (MMR) Gene Mutation Carriers Have Favorable Outcome in Colorectal and Endometrial Cancer: A Prospective Cohort Study.
Yeh JT, Peng HP, Hung FH, Hung CF, Hsieh LL, Yang AS, Wang YA. Yeh JT, et al. Cancers (Basel). 2024 Jun 26;16(13):2342. doi: 10.3390/cancers16132342. Cancers (Basel). 2024. PMID: 39001404 Free PMC article. - Mathematical modeling of multiple pathways in colorectal carcinogenesis using dynamical systems with Kronecker structure.
Haupt S, Zeilmann A, Ahadova A, Bläker H, von Knebel Doeberitz M, Kloor M, Heuveline V. Haupt S, et al. PLoS Comput Biol. 2021 May 18;17(5):e1008970. doi: 10.1371/journal.pcbi.1008970. eCollection 2021 May. PLoS Comput Biol. 2021. PMID: 34003820 Free PMC article.
References
- Møller P, Seppälä T, Bernstein I, et al. . Mallorca Group (http://mallorca-group.org). Incidence of and survival after subsequent cancers in carriers of pathogenic MMR variants with previous cancer: a report from the prospective Lynch syndrome database. Gut 2017;66:1657–64. 10.1136/gutjnl-2016-311403 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous